Explaining distortions in metacognition with an attractor network model of decision uncertainty
- PMID: 34310613
- PMCID: PMC8341696
- DOI: 10.1371/journal.pcbi.1009201
Explaining distortions in metacognition with an attractor network model of decision uncertainty
Abstract
Metacognition is the ability to reflect on, and evaluate, our cognition and behaviour. Distortions in metacognition are common in mental health disorders, though the neural underpinnings of such dysfunction are unknown. One reason for this is that models of key components of metacognition, such as decision confidence, are generally specified at an algorithmic or process level. While such models can be used to relate brain function to psychopathology, they are difficult to map to a neurobiological mechanism. Here, we develop a biologically-plausible model of decision uncertainty in an attempt to bridge this gap. We first relate the model's uncertainty in perceptual decisions to standard metrics of metacognition, namely mean confidence level (bias) and the accuracy of metacognitive judgments (sensitivity). We show that dissociable shifts in metacognition are associated with isolated disturbances at higher-order levels of a circuit associated with self-monitoring, akin to neuropsychological findings that highlight the detrimental effect of prefrontal brain lesions on metacognitive performance. Notably, we are able to account for empirical confidence judgements by fitting the parameters of our biophysical model to first-order performance data, specifically choice and response times. Lastly, in a reanalysis of existing data we show that self-reported mental health symptoms relate to disturbances in an uncertainty-monitoring component of the network. By bridging a gap between a biologically-plausible model of confidence formation and observed disturbances of metacognition in mental health disorders we provide a first step towards mapping theoretical constructs of metacognition onto dynamical models of decision uncertainty. In doing so, we provide a computational framework for modelling metacognitive performance in settings where access to explicit confidence reports is not possible.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

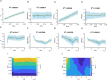
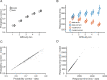


References
-
- Ratcliff R. A theory of memory retrieval. Psychological Review. 1978;85(2):59–108. doi: 10.1037/0033-295x.85.2.59 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

