DENV NS1 and MMP-9 cooperate to induce vascular leakage by altering endothelial cell adhesion and tight junction
- PMID: 34310658
- PMCID: PMC8341711
- DOI: 10.1371/journal.ppat.1008603
DENV NS1 and MMP-9 cooperate to induce vascular leakage by altering endothelial cell adhesion and tight junction
Abstract
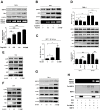
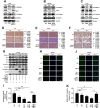
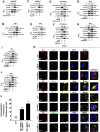
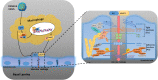
Dengue virus (DENV) is a mosquito-borne pathogen that causes a spectrum of diseases including life-threatening dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS). Vascular leakage is a common clinical crisis in DHF/DSS patients and highly associated with increased endothelial permeability. The presence of vascular leakage causes hypotension, circulatory failure, and disseminated intravascular coagulation as the disease progresses of DHF/DSS patients, which can lead to the death of patients. However, the mechanisms by which DENV infection caused the vascular leakage are not fully understood. This study reveals a distinct mechanism by which DENV induces endothelial permeability and vascular leakage in human endothelial cells and mice tissues. We initially show that DENV2 promotes the matrix metalloproteinase-9 (MMP-9) expression and secretion in DHF patients' sera, peripheral blood mononuclear cells (PBMCs), and macrophages. This study further reveals that DENV non-structural protein 1 (NS1) induces MMP-9 expression through activating the nuclear factor κB (NF-κB) signaling pathway. Additionally, NS1 facilitates the MMP-9 enzymatic activity, which alters the adhesion and tight junction and vascular leakage in human endothelial cells and mouse tissues. Moreover, NS1 recruits MMP-9 to interact with β-catenin and Zona occludens protein-1/2 (ZO-1 and ZO-2) and to degrade the important adhesion and tight junction proteins, thereby inducing endothelial hyperpermeability and vascular leakage in human endothelial cells and mouse tissues. Thus, we reveal that DENV NS1 and MMP-9 cooperatively induce vascular leakage by impairing endothelial cell adhesion and tight junction, and suggest that MMP-9 may serve as a potential target for the treatment of hypovolemia in DSS/DHF patients.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- WHO. Ten threats to global health in 2019 [EB/OL]. https://www.who.int/news-room/feature-stories/ten-threats-to-global-heal....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

