NF-κB Regulation of LRH-1 and ABCG5/8 Potentiates Phytosterol Role in the Pathogenesis of Parenteral Nutrition-Associated Cholestasis
- PMID: 34310734
- PMCID: PMC8639620
- DOI: 10.1002/hep.32071
NF-κB Regulation of LRH-1 and ABCG5/8 Potentiates Phytosterol Role in the Pathogenesis of Parenteral Nutrition-Associated Cholestasis
Abstract
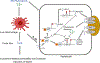
Background and aims: Chronically administered parenteral nutrition (PN) in patients with intestinal failure carries the risk for developing PN-associated cholestasis (PNAC). We have demonstrated that farnesoid X receptor (FXR) and liver X receptor (LXR), proinflammatory interleukin-1 beta (IL-1β), and infused phytosterols are important in murine PNAC pathogenesis. In this study we examined the role of nuclear receptor liver receptor homolog 1 (LRH-1) and phytosterols in PNAC.
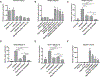
Approach and results: In a C57BL/6 PNAC mouse model (dextran sulfate sodium [DSS] pretreatment followed by 14 days of PN; DSS-PN), hepatic nuclear receptor subfamily 5, group A, member 2/LRH-1 mRNA, LRH-1 protein expression, and binding of LRH-1 at the Abcg5/8 and Cyp7a1 promoter was reduced. Interleukin-1 receptor-deficient mice (Il-1r-/- /DSS-PN) were protected from PNAC and had significantly increased hepatic mRNA and protein expression of LRH-1. NF-κB activation and binding to the LRH-1 promoter were increased in DSS-PN PNAC mice and normalized in Il-1r-/- /DSS-PN mice. Knockdown of NF-κB in IL-1β-exposed HepG2 cells increased expression of LRH-1 and ABCG5. Treatment of HepG2 cells and primary mouse hepatocytes with an LRH-1 inverse agonist, ML179, significantly reduced mRNA expression of FXR targets ATP binding cassette subfamily C member 2/multidrug resistance associated protein 2 (ABCC2/MRP2), nuclear receptor subfamily 0, groupB, member 2/small heterodimer partner (NR0B2/SHP), and ATP binding cassette subfamily B member 11/bile salt export pump (ABCB11/BSEP). Co-incubation with phytosterols further reduced expression of these genes. Similar results were obtained by suppressing the LRH-1 targets ABCG5/8 by treatment with small interfering RNA, IL-1β, or LXR antagonist GSK2033. Liquid chromatography-mass spectrometry and chromatin immunoprecipitation experiments in HepG2 cells showed that ATP binding cassette subfamily G member 5/8 (ABCG5/8) suppression by GSK2033 increased the accumulation of phytosterols and reduced binding of FXR to the SHP promoter. Finally, treatment with LRH-1 agonist, dilauroyl phosphatidylcholine (DLPC) protected DSS-PN mice from PNAC.
Conclusions: This study suggests that NF-κB regulation of LRH-1 and downstream genes may affect phytosterol-mediated antagonism of FXR signaling in the pathogenesis of PNAC. LRH-1 could be a potential therapeutic target for PNAC.
© 2021 by the American Association for the Study of Liver Diseases.
Conflict of interest statement
Potential conflict of interest: Dr. Sokol consults for Mirum and Albireo. Dr. D’Alessandro consults for Rubius. He advises Hemanext and Forma. He owns stock in Omix and Altis.
Figures




References
-
- Orso G, Mandato C, Veropalumbo C, Cecchi N, Garzi A, Vajro P. Pediatric parenteral nutrition-associated liver disease and cholestasis: novel advances in pathomechanisms-based prevention and treatment. Dig Liver Dis 2016;48:215–222. - PubMed
-
- Kelly DA. Intestinal failure-associated liver disease: what do we know today? Gastroenterology 2006;130:S70–S77. - PubMed
-
- Ganousse-Mazeron S, Lacaille F, Colomb-Jung V, Talbotec C, Ruemmele F, Sauvat F, et al. Assessment and outcome of children with intestinal failure referred for intestinal transplantation. Clin Nutr 2015;34:428–435. - PubMed
-
- Israelite JC. Pediatric parenteral nutrition-associated liver disease. J Infus Nurs 2017;40:51–54. - PubMed
-
- Christensen RD, Henry E, Wiedmeier SE, Burnett J, Lambert DK. Identifying patients, on the first day of life, at high-risk of developing parenteral nutrition-associated liver disease. J Perinatol 2007;27:284–290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

