Efficacy and safety of CD19-directed CAR-T cell therapies in patients with relapsed/refractory aggressive B-cell lymphomas: Observations from the JULIET, ZUMA-1, and TRANSCEND trials
- PMID: 34310745
- PMCID: PMC9290945
- DOI: 10.1002/ajh.26301
Efficacy and safety of CD19-directed CAR-T cell therapies in patients with relapsed/refractory aggressive B-cell lymphomas: Observations from the JULIET, ZUMA-1, and TRANSCEND trials
Abstract
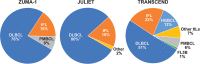
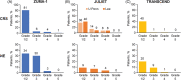
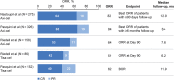
Chimeric antigen receptor (CAR)-T cell therapies have improved the outcome for many patients with relapsed or refractory aggressive B-cell lymphomas. In 2017, axicabtagene ciloleucel and soon after tisagenlecleucel became the first approved CAR-T cell products for patients with high-grade B-cell lymphomas or diffuse large B-cell lymphoma (DLBCL) who are relapsed or refractory to ≥ 2 prior lines of therapy; lisocabtagene maraleucel was approved in 2021. Safety and efficacy outcomes from the pivotal trials of each CAR-T cell therapy have been reported. Despite addressing a common unmet need in the large B-cell lymphoma population and utilizing similar CAR technologies, there are differences between CAR-T cell products in manufacturing, pivotal clinical trial designs, and data reporting. Early reports of commercial use of axicabtagene ciloleucel and tisagenlecleucel provide the first opportunities to validate the impact of patient characteristics on the efficacy and safety of these CAR-T cell therapies in the real world. Going forward, caring for patients after CAR-T cell therapy will require strategies to monitor patients for sustained responses and potential long-term side effects. In this review, product attributes, protocol designs, and clinical outcomes of the key clinical trials are presented. We discuss recent data on patient characteristics, efficacy, and safety of patients treated with axicabtagene ciloleucel or tisagenlecleucel in the real world. Finally, we discuss postinfusion management and preview upcoming clinical trials of CAR-T cell therapies.
Trial registration: ClinicalTrials.gov NCT02445248 NCT02348216 NCT02631044.
© 2021 The Authors. American Journal of Hematology published by Wiley Periodicals LLC.
Conflict of interest statement
Jason R. Westin is an advisor or consultant for Celgene, Curis, Genentech, Janssen, Juno, Kite, MorphoSys, and Novartis; and reports research support from Celgene, Curis, Forty Seven Inc, Genentech, Janssen, Kite, Novartis, and Unum. Marie José Kersten is an advisor or consultant for, and reports honoraria and travel support from Amgen, Bristol‐Myers Squibb, Celgene, Janssen, Kite/Gilead, Miltenyi Biotec, MSD, Novartis, and Roche; and research support from Celgene, Roche, Kite/Gilead, and Takeda. Gilles Salles is an advisor or consultant for AbbVie, Allogene, Autolus, BeiGene, Celgene, Epizyme, Genmab, Gilead, Janssen, Kite, Merck, MorphoSys, Novartis, Roche, and VelosBio; reports honoraria from AbbVie, Amgen, Bristol‐Myers Squibb, Celgene, Epizyme, Gilead, Janssen, Kite, Merck, MorphoSys, Novartis, and Roche; and reports participation in educational events for AbbVie, Celgene, Gilead, Janssen, Kite, MorphoSys, Novartis, and Roche. Jeremy S. Abramson is an advisor or consultant for Celgene, Genentech/Roche, Gilead Sciences, Novartis, and Seattle Genetics; and reports research funding from Celgene (Inst), Genentech (Inst), Millennium (Inst), and Seattle Genetics (Inst). Stephen J. Schuster is an advisor or consultant for Acerta, Allogene, AstraZeneca, BeiGene, Celgene/Juno, Genentech/Roche, Loxo Oncology, Novartis, and Tessa Therapeutics; reports honoraria from Acerta, Allogene, AstraZeneca, BeiGene, Celgene, Genentech/Roche, Loxo Oncology, Novartis, Nordic Nanovector, Pfizer, and Tessa Therapeutics; reports steering committee participation for AbbVie, Celgene, Novartis, Juno, Nordic Nanovector, and Pfizer; reports research support from AbbVie, Acerta, Celgene/Juno, DTRM Bio, Genentech, Incyte, Merck, Novartis, Portola, and TG therapeutics; and holds a patent with Novartis. Frederick L. Locke is a scientific advisor for Allogene, Amgen, BlueBird Bio, BMS/Celgene, Calibr, GammaDelta Therapeutics, Iovance, Janssen, Kite Pharma, Legend, Novartis, and Wugen; reports consultancy for Cellular Biomedicine Group Inc; reports research funding from Allogene (Inst), Kite (Inst), and Novartis (Inst); and reports his institution holding unlicensed patents in his name, in the field of cellular immunotherapy. Charalambos Andreadis is an advisor or consultant for Genentech, Gilead Sciences/Kite Pharma, and Karyopharm; reports honoraria from Bristol‐Myers Squibb/Celgene/Juno, and Jazz Pharmaceuticals; reports research funding from Bristol‐Myers Squibb/Celgene/Juno, Incyte, Merck, and Novartis; and is a current equity holder in Genentech.
Figures
References
-
- Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B‐cell lymphoma. N Engl J Med. 2019;380(1):45‐56. - PubMed
