"Golden" Cascade Cyclization to Benzo[c]-Phenanthridines
- PMID: 34310792
- PMCID: PMC8597101
- DOI: 10.1002/chem.202102134
"Golden" Cascade Cyclization to Benzo[c]-Phenanthridines
Abstract
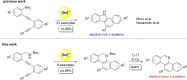
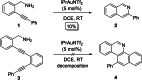
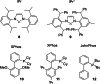
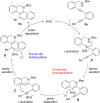
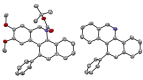
Herein, we describe a gold-catalyzed cascade cyclization of Boc-protected benzylamines bearing two tethered alkyne moieties in a domino reaction initiated by a 6-endo-dig cyclization. The reaction was screened intensively, and the scope was explored, resulting in nine new Boc-protected dihydrobenzo[c]phenanthridines with yields of up to 98 %; even a π-extension and two bidirectional approaches were successful. Furthermore, thermal cleavage of the Boc group and subsequent oxidation gave substituted benzo[c]phenanthridines in up to quantitative yields. Two bidirectional approaches under the optimized conditions were successful, and the resulting π-extended molecules were tested as organic semiconductors in organic thin-film transistors.
Keywords: benzo[c]phenanthridines; benzylamines; cascade reactions; homogeneous gold catalysis.
© 2021 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
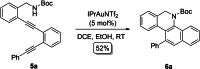

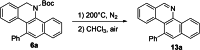
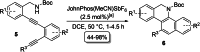
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

