Plasmodium Preerythrocytic Vaccine Antigens Enhance Sterile Protection in Mice Induced by Circumsporozoite Protein
- PMID: 34310889
- PMCID: PMC8519292
- DOI: 10.1128/IAI.00165-21
Plasmodium Preerythrocytic Vaccine Antigens Enhance Sterile Protection in Mice Induced by Circumsporozoite Protein
Abstract
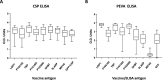
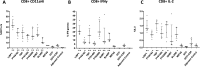
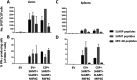
Preerythrocytic vaccines prevent malaria by targeting parasites in the clinically silent sporozoite and liver stages and preventing progression to the virulent blood stages. The leading preerythrocytic vaccine, RTS,S/AS01E (Mosquirix), entered implementation programs in 2019 and targets the major sporozoite surface antigen, circumsporozoite protein (CSP). However, in phase III clinical trials, RTS,S conferred partial protection with limited durability, indicating a need to improve CSP-based vaccination. Previously, we identified highly expressed liver-stage proteins that could potentially be used in combination with CSP; they are referred to as preerythrocytic vaccine antigens (PEVAs). Here, we developed heterologous prime-boost CSP vaccination models to confer partial sterilizing immunity against Plasmodium yoelii (protein prime-adenovirus 5 [Ad5] boost) and Plasmodium berghei (DNA prime-Ad5 boost) in mice. When combined as individual antigens with P. yoelii CSP (PyCSP), three of eight P. yoelii PEVAs significantly enhanced sterile protection against sporozoite challenge, compared to PyCSP alone. Similar results were obtained when three P. berghei PEVAs and P. berghei CSP were combined in a single vaccine regimen. In general, PyCSP antibody responses were similar after CSP alone versus CSP plus PEVA vaccinations. Both P. yoelii and P. berghei CSP plus PEVA combination vaccines induced robust CD8+ T cell responses, including signature gamma interferon (IFN-γ) increases. In the P. berghei model system, IFN-γ responses were significantly higher in hepatic versus splenic CD8+ T cells. The addition of novel antigens may enhance the degree and duration of sterile protective immunity conferred by a human vaccine such as RTS,S.
Keywords: circumsporozoite protein; malaria; preerythrocytic; sterile protection; vaccine.
Figures


References
-
- World Health Organization. 2020. World malaria report 2020. World Health Organization, Geneva, Switzerland.
-
- Speake C, Pichugin A, Sahu T, Malkov V, Morrison R, Pei Y, Juompan L, Milman N, Zarling S, Anderson C, Wong-Madden S, Wendler J, Ishizuka A, MacMillen ZW, Garcia V, Kappe SHI, Krzych U, Duffy PE. 2016. Identification of novel pre-erythrocytic malaria antigen candidates for combination vaccines with circumsporozoite protein. PLoS One 11:e0159449. 10.1371/journal.pone.0159449. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

