Pre-incubation with OATP1B1 and OATP1B3 inhibitors potentiates inhibitory effects in physiologically relevant sandwich-cultured primary human hepatocytes
- PMID: 34311070
- PMCID: PMC11005446
- DOI: 10.1016/j.ejps.2021.105951
Pre-incubation with OATP1B1 and OATP1B3 inhibitors potentiates inhibitory effects in physiologically relevant sandwich-cultured primary human hepatocytes
Abstract
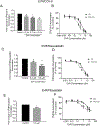
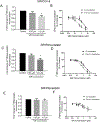
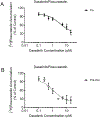
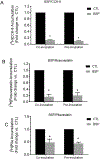
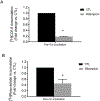
Organic anion transporting polypeptides (OATP)1B1 and OATP1B3 are liver-specific transport proteins that express on the basolateral membrane of human hepatocytes and mediate hepatic uptake of many drugs, including statins. They are important determinants of transporter-mediated drug-drug interactions (DDIs). It has been reported that pre-incubation with some OATP1B1 and OATP1B3 inhibitors potentiates the inhibitory effects, yielding reduced IC50 values. The US FDA draft guidance has recently recommended to use the lower IC50 values after inhibitor-preincubation to assess OATP1B1 and OATP1B3-mediated DDIs. However, it remains unknown whether the potentiation effects of inhibitor-preincubation on IC50 values occur in a physiologically relevant cell model. The current study was designed to determine the IC50 values of OATP1B1 and OATP1B3 inhibitors everolimus (EVR), sirolimus (SIR), and dasatinib against OATP1B substrates in physiologically relevant primary human hepatocytes with or without inhibitor-preincubation and to compare the OATP-mediated DDI prediction using data from primary human hepatocytes and that reported previously in transporter-expressing cell lines. Primary human hepatocytes were cultured in a sandwich configuration. Accumulation of [3H]-CCK-8 (1 µM, 1.5 min), [3H]-rosuvastatin (0.5 µM, 2 min) and [3H]-pitavastatin (1 µM, 0.5 min) was determined in human sandwich-cultured hepatocytes (SCH) in the presence of vehicle control or an inhibitor with or without inhibitor-preincubation at designated concentrations, and was utilized to determine the IC50 values for these inhibitors. R-value models were used to predict OATP-mediated DDIs. Pre-incubation with EVR at a clinically relevant concentration of 0.2 µM significantly reduced accumulation of [3H]-CCK-8 and [3H]-rosuvastatin even after washing. Reduced IC50 values following inhibitor pre-incubation were observed for all three inhibitors using [3H]-CCK-8 and [3H]-rosuvastatin as substrates in human SCH. The IC50 values after inhibitor-preincubation were lower or comparable in transporter-expressing cell lines compared with that in human SCH. For dasatinib, R-values from both cell lines and human SCH were greater than the US FDA cut-off value of 1.1. For EVR, R values from cell lines were 1.23 and were lowered to near 1.1 (1.08-1.09) in human SCH. For SIR, R values from either cell type were less than the cut-off values of 1.1. In conclusion, the current study is the first to report that pre-incubation with OATP1B inhibitors potentiates inhibitory effects in physiologically relevant primary human hepatocytes, supporting the rationale of the current US FDA draft guidance of including an inhibitor-preincubation step when assessing OATP-mediated DDIs in vitro. IC50 values after inhibitor-preincubation in transporter-expressing cell lines may be used for DDI prediction for the purpose of mitigating false-negative OATP-mediated DDI prediction.
Keywords: Drug interactions; Drug transport; Hepatic transport; Hepatocytes; Organic anion-transporting polypeptide; Pharmacokinetics; Physiologically based pharmacokinetic modeling; Transporters.
Copyright © 2021 Elsevier B.V. All rights reserved.
Figures
References
-
- Amundsen R, Christensen H, Zabihyan B, and Asberg A (2010) Cyclosporine A, but not tacrolimus, shows relevant inhibition of organic anion-transporting protein 1B1-mediated transport of atorvastatin. Drug Metab Dispos 38:1499–1504. - PubMed
-
- Annaert P, Ye ZW, Stieger B, and Augustijns P (2010) Interaction of HIV protease inhibitors with OATP1B1, 1B3, and 2B1. Xenobiotica 40:163–176. - PubMed
-
- Bi YA, Qiu X, Rotter CJ, Kimoto E, Piotrowski M, Varma MV, Ei-Kattan AF, and Lai Y (2013) Quantitative assessment of the contribution of sodium-dependent taurocholate co-transporting polypeptide (NTCP) to the hepatic uptake of rosuvastatin, pitavastatin and fluvastatin. Biopharm Drug Dispos 34:452–461. - PubMed
-
- Bradley SE, Ingelfinger FJ, and et al. (1945) The estimation of hepatic blood flow in man. The Journal of clinical investigation 24:890–897. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

