Understanding the benefits and burdens associated with a malaria human infection study in Kenya: experiences of study volunteers and other stakeholders
- PMID: 34311781
- PMCID: PMC8313115
- DOI: 10.1186/s13063-021-05455-7
Understanding the benefits and burdens associated with a malaria human infection study in Kenya: experiences of study volunteers and other stakeholders
Abstract
Background: Human infection studies (HIS) that involve deliberately infecting healthy volunteers with a pathogen raise important ethical issues, including the need to ensure that benefits and burdens are understood and appropriately accounted for. Building on earlier work, we embedded social science research within an ongoing malaria human infection study in coastal Kenya to understand the study benefits and burdens experienced by study stakeholders in this low-resource setting and assess the wider implications for future research planning and policy.
Methods: Data were collected using qualitative research methods, including in-depth interviews (44), focus group discussions (10) and non-participation observation. Study participants were purposively selected (key informant or maximal diversity sampling), including volunteers in the human infection study, study staff, community representatives and local administrative authorities. Data were collected during and up to 18 months following study residency, from sites in Coastal and Western Kenya. Voice recordings of interviews and discussions were transcribed, translated, and analysed using framework analysis, combining data- and theory-driven perspectives.
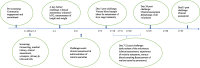
Findings: Physical, psychological, economic and social forms of benefits and burdens were experienced across study stages. Important benefits for volunteers included the study compensation, access to health checks, good residential living conditions, new learning opportunities, developing friendships and satisfaction at contributing towards a new malaria vaccine. Burdens primarily affected study volunteers, including experiences of discomfort and ill health; fear and anxiety around aspects of the trial process, particularly deliberate infection and the implications of prolonged residency; anxieties about early residency exit; and interpersonal conflict. These issues had important implications for volunteers' families, study staff and the research institution's reputation more widely.
Conclusion: Developing ethically and scientifically strong HIS relies on grounded accounts of volunteers, study staff and the wider community, understood in the socioeconomic, political and cultural context where studies are implemented. Recognition of the diverse, and sometimes perverse, nature of potential benefits and burdens in a given context, and who this might implicate, is critical to this process. Prior and ongoing stakeholder engagement is core to developing these insights.
Keywords: Benefits; Burdens; Challenge studies; Controlled human infection studies; Developing countries; Ethics; Human infection studies.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Shah SK, Kimmelman J, Lyerly AD, Lynch HF, McCutchan F, Miller F, et al. Ethical Considerations for Zika Virus Human Challenge Trials: Report & Recommendations. National Institute of Allergy and Infectious Diseases. February 2017. https://www.niaid.nih.gov/sites/default/files/EthicsZikaHumanChallengeSt.... Accessed 17 Nov 2020.
-
- Kapulu MC, Njuguna P, Hamaluba MM, CHMI-SIKA Study Team Controlled human malaria infection in semi-ommune Kenyan adults (CHMI-SIKA): a study protocol to investigate in vivo Plasmodium falciparum malaria parasite growth in the context of pre-existing immunity. Wellcome Open Res. 2019;3:155. doi: 10.12688/wellcomeopenres.14909.2. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

