Humoral Response to mRNA versus an Adenovirus Vector-Based SARS-CoV-2 Vaccine in Dialysis Patients
- PMID: 34312162
- PMCID: PMC8729415
- DOI: 10.2215/CJN.06450521
Humoral Response to mRNA versus an Adenovirus Vector-Based SARS-CoV-2 Vaccine in Dialysis Patients
Keywords: COVID-19; SARS-CoV-2; antibody; dialysis; vaccination.
Figures
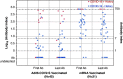
References
-
- Siemens Healthcare Diagnostics : ADVIA Centaur XP/XPT S ARS-CoV-2 IgG (COV2G) Instructions for use. Available at: https://www.fda.gov/media/140704/download. Accessed April 18, 2021
-
- US Food and Drug Administration : Investigational COVID-19 convalescent plasma: Guidance for industry. May 1, 2020. Available at: https://www.hhs.gov/guidance/document/investigational-covid-19-convalesc.... Accessed May 3, 2021
-
- Conklin J, Freeman J, Patel S, Vero L, Pennamon G, Glatz J: Evaluation of the enhanced SARS-CoV-2 IgG (sCOVG) assay* on the Atellica IM Analyzer. Poster presented at: The International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) Virtual Conference on Critical Role of Clinical Laboratories in the Covid-19 Pandemic; February 15–17, 2021
-
- Stephenson KE, Le Gars M, Sadoff J, de Groot AM, Heerwegh D, Truyers C, Atyeo C, Loos C, Chandrashekar A, McMahan K, Tostanoski LH, Yu J, Gebre MS, Jacob-Dolan C, Li Z, Patel S, Peter L, Liu J, Borducchi EN, Nkolola JP, Souza M, Tan CS, Zash R, Julg B, Nathavitharana RR, Shapiro RL, Azim AA, Alonso CD, Jaegle K, Ansel JL, Kanjilal DG, Guiney CJ, Bradshaw C, Tyler A, Makoni T, Yanosick KE, Seaman MS, Lauffenburger DA, Alter G, Struyf F, Douoguih M, Van Hoof J, Schuitemaker H, Barouch DH: Immunogenicity of the Ad26.COV2.S vaccine for COVID-19. JAMA 325: 1535–1544, 2021 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

