Efficacy and safety of bridging thrombolysis initiated before transfer in a drip-and-ship stroke service
- PMID: 34312320
- PMCID: PMC8899648
- DOI: 10.1136/svn-2021-001024
Efficacy and safety of bridging thrombolysis initiated before transfer in a drip-and-ship stroke service
Abstract
Objective: Data regarding the efficacy and safety of bridging thrombolysis (BT) initiated before transfer for evaluation of endovascular therapy is heterogeneous. We, therefore, analyse efficacy and safety of BT in patients treated within a drip-and-ship stroke service.
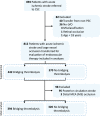
Methods: Consecutive adult patients suffering from acute ischaemic stroke and large-vessel occlusions (LVO) transferred to our comprehensive stroke centre for evaluation of endovascular therapy in 2017-2020 were identified from a local prospective stroke database and categorised according to BT and no-BT. BT was defined as intravenous thrombolysis initiated before transfer. LVO was assessed before and after transfer. Functional outcome before stroke and at 3 months using the modified Rankin scale (mRS) was determined. Excellent outcome was defined as mRS 0-1 or return to prestroke mRS. For safety analysis, intracranial haemorrhages and mortality at 3 months were analysed. Main analysis was limited to patients with anterior circulation stroke.
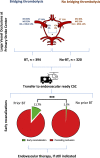
Results: Of N=714 patients, n=394 (55.2%) received BT. More patients in the BT group with documented LVO before transfer recanalised without endovascular therapy (n=46, 11.7%) than patients who did not receive BT before transfer (n=4, 1.3%, p<0.001). In multivariate analysis, BT was the strongest independent predictor of early recanalisation (adjusted OR 10.9, 95% CI 3.8 to 31.1, p<0.001). BT tended to be an independent predictor of an excellent outcome at 3 months (adjusted OR 1.38, 95% CI 0.97 to 1.96, p=0.077). There were no differences in safety between the BT and no-BT groups.
Conclusions: BT initiated before transfer was a strong independent predictor of early recanalisation.
Keywords: stroke; thrombectomy; thrombolysis.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JCP has received consultation fees and travel expenses from Akcea, Bayer, Boehringer Ingelheim, Daiichi Sankyo and Pfizer. MM reports grants/grants pending from Balt, Medtronic, MicroVention, Stryker (money paid to the institution). PAR received travel support and lecture fees from Boeheringer Ingelheim, Daiichi Sankyo, Pfizer, and Bayer. SN has received consulting fees from Brainomix and Boehringer Ingelheim and lecture fees and travel expenses from Medtronic and Pfizer. CG is speaker of the commission telemedicine service of the German Stroke Society.
Figures
References
-
- Pérez de la Ossa N, Abilleira S, Jiménez X, eds. Transfer to the Local Stroke Center versus Direct Transfer to Endovascular Center of Acute Stroke Patients with Suspected Large Vessel Occlusion in the Cataln Territory (RACECAT). European Stroke Organisation Conference, 2020. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
