Extracellular NLRP3 inflammasome particles are internalized by human coronary artery smooth muscle cells and induce pro-atherogenic effects
- PMID: 34312415
- PMCID: PMC8313534
- DOI: 10.1038/s41598-021-94314-1
Extracellular NLRP3 inflammasome particles are internalized by human coronary artery smooth muscle cells and induce pro-atherogenic effects
Abstract
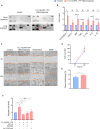
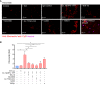
Inflammation driven by intracellular activation of the NLRP3 inflammasome is involved in the pathogenesis of a variety of diseases including vascular pathologies. Inflammasome specks are released into the extracellular compartment from disrupting pyroptotic cells. The potential uptake and function of extracellular NLRP3 inflammasomes in human coronary artery smooth muscle cells (HCASMC) are unknown. Fluorescently labeled NLRP3 inflammasome particles were isolated from a mutant NLRP3-YFP cell line and used to treat primary HCASMC for 4 and 24 h. Fluorescent and expressional analyses showed that extracellular NLRP3-YFP particles are internalized into HCASMC, where they remain active and stimulate intracellular caspase-1 (1.9-fold) and IL-1β (1.5-fold) activation without inducing pyroptotic cell death. Transcriptomic analysis revealed increased expression level of pro-inflammatory adhesion molecules (ICAM1, CADM1), NLRP3 and genes involved in cytoskleleton organization. The NLRP3-YFP particle-induced gene expression was not dependent on NLRP3 and caspase-1 activation. Instead, the effects were partly abrogated by blocking NFκB activation. Genes, upregulated by extracellular NLRP3 were validated in human carotid artery atheromatous plaques. Extracellular NLRP3-YFP inflammasome particles promoted the secretion of pro-atherogenic and inflammatory cytokines such as CCL2/MCP1, CXCL1 and IL-17E, and increased HCASMC migration (1.8-fold) and extracellular matrix production, such as fibronectin (5.8-fold) which was dependent on NFκB and NLRP3 activation. Extracellular NLRP3 inflammasome particles are internalized into human coronary artery smooth muscle cells where they induce pro-inflammatory and pro-atherogenic effects representing a novel mechanism of cell-cell communication and perpetuation of inflammation in atherosclerosis. Therefore, extracellular NLRP3 inflammasomes may be useful to improve the diagnosis of inflammatory diseases and the development of novel anti-inflammatory therapeutic strategies.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

