Faecal biomarkers in type 1 diabetes with and without diabetic nephropathy
- PMID: 34312454
- PMCID: PMC8313679
- DOI: 10.1038/s41598-021-94747-8
Faecal biomarkers in type 1 diabetes with and without diabetic nephropathy
Abstract
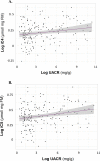
Gastrointestinal dysbiosis is common among persons with type 1 diabetes (T1D), but its potential impact on diabetic nephropathy (DN) remains obscure. We examined whether faecal biomarkers, previously associated with low-grade gastrointestinal inflammation, differ between healthy controls and T1D subjects with and without DN. Faecal samples were analyzed for levels of calprotectin, intestinal alkaline phosphatase (IAP), short-chain fatty acids (SCFA) and immunoglobulins in subjects with T1D (n = 159) and healthy controls (NDC; n = 50). The subjects with T1D were stratified based on albuminuria: normoalbuminuria (< 30 mg/g; n = 49), microalbuminuria (30-299 mg/g; n = 50) and macroalbuminuria (≥ 300 mg/g; n = 60). aecal calprotectin, IAP and immunoglobulin levels did not differ between the T1D albuminuria groups. However, when subjects were stratified based on faecal calprotectin cut-off level (50 µg/g), macroalbuminuric T1D subjects exceeded the threshold more frequently than NDC (p = 0.02). Concentrations of faecal propionate and butyrate were lower in T1D subjects compared with NDC (p = 0.04 and p = 0.03, respectively). Among T1D subjects, levels of branched SCFA (BCFA) correlated positively with current albuminuria level (isobutyrate, p = 0.03; isovalerate, p = 0.005). In our study cohort, fatty acid metabolism seemed to be altered among T1D subjects and those with albuminuria compared to NDC. This may reflect gastrointestinal imbalances associated with T1D and renal complications.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

