ChAdOx1 nCoV-19 protection against SARS-CoV-2 in rhesus macaque and ferret challenge models
- PMID: 34312487
- PMCID: PMC8313674
- DOI: 10.1038/s42003-021-02443-0
ChAdOx1 nCoV-19 protection against SARS-CoV-2 in rhesus macaque and ferret challenge models
Abstract
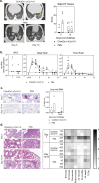
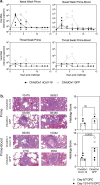
Vaccines against SARS-CoV-2 are urgently required, but early development of vaccines against SARS-CoV-1 resulted in enhanced disease after vaccination. Careful assessment of this phenomena is warranted for vaccine development against SARS CoV-2. Here we report detailed immune profiling after ChAdOx1 nCoV-19 (AZD1222) and subsequent high dose challenge in two animal models of SARS-CoV-2 mediated disease. We demonstrate in rhesus macaques the lung pathology caused by SARS-CoV-2 mediated pneumonia is reduced by prior vaccination with ChAdOx1 nCoV-19 which induced neutralising antibody responses after a single intramuscular administration. In a second animal model, ferrets, ChAdOx1 nCoV-19 reduced both virus shedding and lung pathology. Antibody titre were boosted by a second dose. Data from these challenge models on the absence of enhanced disease and the detailed immune profiling, support the continued clinical evaluation of ChAdOx1 nCoV-19.
© 2021. The Author(s).
Conflict of interest statement
SCG is co-founder and board member of Vaccitech (collaborators in the early development of this vaccine candidate) and named as an inventor on a patent covering the use of ChAdOx1-vectored vaccines and a patent application covering this SARS-CoV-2 vaccine. TL is named as an inventor on a patent application covering this SARS-CoV-2 vaccine and consultant to Vaccitech. All other authors had no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

