Mucosal vaccines - fortifying the frontiers
- PMID: 34312520
- PMCID: PMC8312369
- DOI: 10.1038/s41577-021-00583-2
Mucosal vaccines - fortifying the frontiers
Erratum in
-
Publisher Correction: Mucosal vaccines - fortifying the frontiers.Nat Rev Immunol. 2022 Apr;22(4):266. doi: 10.1038/s41577-021-00599-8. Nat Rev Immunol. 2022. PMID: 34345023 Free PMC article. No abstract available.
Abstract
Mucosal vaccines offer the potential to trigger robust protective immune responses at the predominant sites of pathogen infection. In principle, the induction of adaptive immunity at mucosal sites, involving secretory antibody responses and tissue-resident T cells, has the capacity to prevent an infection from becoming established in the first place, rather than only curtailing infection and protecting against the development of disease symptoms. Although numerous effective mucosal vaccines are in use, the major advances seen with injectable vaccines (including adjuvanted subunit antigens, RNA and DNA vaccines) have not yet been translated into licensed mucosal vaccines, which currently comprise solely live attenuated and inactivated whole-cell preparations. The identification of safe and effective mucosal adjuvants allied to innovative antigen discovery and delivery strategies is key to advancing mucosal vaccines. Significant progress has been made in resolving the mechanisms that regulate innate and adaptive mucosal immunity and in understanding the crosstalk between mucosal sites, and this provides valuable pointers to inform mucosal adjuvant design. In particular, increased knowledge on mucosal antigen-presenting cells, innate lymphoid cell populations and resident memory cells at mucosal sites highlights attractive targets for vaccine design. Exploiting these insights will allow new vaccine technologies to be leveraged to facilitate rational mucosal vaccine design for pathogens including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and for cancer.
© 2021. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures
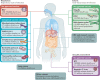


References
-
- WHO. The top 10 causes of death. World Health Organizationhttps://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (2020).
-
- Troeger C, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018;18:1191–1210. doi: 10.1016/S1473-3099(18)30310-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

