DNA-loop-extruding SMC complexes can traverse one another in vivo
- PMID: 34312537
- PMCID: PMC8878250
- DOI: 10.1038/s41594-021-00626-1
DNA-loop-extruding SMC complexes can traverse one another in vivo
Abstract
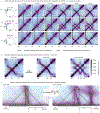
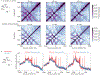
Chromosome organization mediated by structural maintenance of chromosomes (SMC) complexes is vital in many organisms. SMC complexes act as motors that extrude DNA loops, but it remains unclear what happens when multiple complexes encounter one another on the same DNA in living cells and how these interactions may help to organize an active genome. We therefore created a crash-course track system to study SMC complex encounters in vivo by engineering defined SMC loading sites in the Bacillus subtilis chromosome. Chromosome conformation capture (Hi-C) analyses of over 20 engineered strains show an amazing variety of chromosome folding patterns. Through three-dimensional polymer simulations and theory, we determine that these patterns require SMC complexes to bypass each other in vivo, as recently seen in an in vitro study. We posit that the bypassing activity enables SMC complexes to avoid traffic jams while spatially organizing the genome.
© 2021. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures
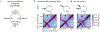



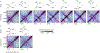









References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

