Optimal design of cluster randomized trials allowing unequal allocation of clusters and unequal cluster size between arms
- PMID: 34312902
- PMCID: PMC7614658
- DOI: 10.1002/sim.9135
Optimal design of cluster randomized trials allowing unequal allocation of clusters and unequal cluster size between arms
Abstract
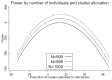
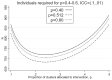
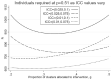
There are sometimes cost, scientific, or logistical reasons to allocate individuals unequally in an individually randomized trial. In cluster randomized trials we can allocate clusters unequally and/or allow different cluster size between trial arms. We consider parallel group designs with a continuous outcome, and optimal designs that require the smallest number of individuals to be measured given the number of clusters. Previous authors have derived the optimal allocation ratio for clusters under different variance and/or intracluster correlations (ICCs) between arms, allowing different but prespecified cluster sizes by arm. We derive closed-form expressions to identify the optimal proportions of clusters and of individuals measured for each arm, thereby defining optimal cluster sizes, when cluster size can be chosen freely. When ICCs differ between arms but the variance is equal, the optimal design allocates more than half the clusters to the arm with the higher ICC, but (typically only slightly) less than half the individuals and hence a smaller cluster size. We also describe optimal design under constraints on the number of clusters or cluster size in one or both arms. This methodology allows trialists to consider a range for the number of clusters in the trial and for each to identify the optimal design. Except if there is clear prior evidence for the ICC and variance by arm, a range of values will need to be considered. Researchers should choose a design with adequate power across the range, while also keeping enough clusters in each arm to permit the intended analysis method.
Keywords: cluster randomized trial; optimal design; power; sample size; unequal allocation.
© 2021 The Authors. Statistics in Medicine published by John Wiley & Sons Ltd.
Figures
References
-
- Gail M, Williams R, Byar DP, Brown C. How many controls? J Chron Dis. 1976;29:723–731. - PubMed
-
- Roberts C, Roberts SA. Design and analysis of clinical trials with clustering effects due to treatment. Clinical Trials. 2005;2:152–162. - PubMed
-
- Candel MJJM, Van Breukelen GJP. Sample size calculation for treatment effects in randomized trials with fixed cluster sizes and heterogeneous intraclass correlations and variances. Stat Meth Med Res. 2015;24(5):557–573. - PubMed
-
- Moerbeek M, Wong WK. Sample size formulae for trials comparing group and individual treatments in a multilevel model. Stat Med. 2008;27:2850–2864. - PubMed
-
- Perkins GD, Lall R, Quinn T, et al. Mechanical versus manual chest compression for out-of-hospital cardiac arrest (PARAMEDIC): a pragmatic, cluster randomised controlled trial. Lancet. 2015;385:947–955. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
