Perception of microstimulation frequency in human somatosensory cortex
- PMID: 34313221
- PMCID: PMC8376245
- DOI: 10.7554/eLife.65128
Perception of microstimulation frequency in human somatosensory cortex
Abstract
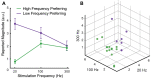
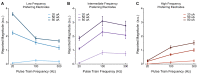
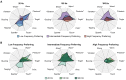
Microstimulation in the somatosensory cortex can evoke artificial tactile percepts and can be incorporated into bidirectional brain-computer interfaces (BCIs) to restore function after injury or disease. However, little is known about how stimulation parameters themselves affect perception. Here, we stimulated through microelectrode arrays implanted in the somatosensory cortex of two human participants with cervical spinal cord injury and varied the stimulus amplitude, frequency, and train duration. Increasing the amplitude and train duration increased the perceived intensity on all tested electrodes. Surprisingly, we found that increasing the frequency evoked more intense percepts on some electrodes but evoked less-intense percepts on other electrodes. These different frequency-intensity relationships were divided into three groups, which also evoked distinct percept qualities at different stimulus frequencies. Neighboring electrode sites were more likely to belong to the same group. These results support the idea that stimulation frequency directly controls tactile perception and that these different percepts may be related to the organization of somatosensory cortex, which will facilitate principled development of stimulation strategies for bidirectional BCIs.
Keywords: brain-computer interfaces; human; intracortical microstimulation; neuroscience; sensory feedback; somatosensory cortex.
Conflict of interest statement
CH, SF, JW, MB, JC, RG No competing interests declared
Figures









References
-
- Anselin L. Local indicators of spatial Association-LISA. Geographical Analysis. 1995;27:93–115. doi: 10.1111/j.1538-4632.1995.tb00338.x. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

