Preclinical characterization of dostarlimab, a therapeutic anti-PD-1 antibody with potent activity to enhance immune function in in vitro cellular assays and in vivo animal models
- PMID: 34313545
- PMCID: PMC8317941
- DOI: 10.1080/19420862.2021.1954136
Preclinical characterization of dostarlimab, a therapeutic anti-PD-1 antibody with potent activity to enhance immune function in in vitro cellular assays and in vivo animal models
Abstract
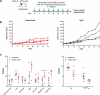
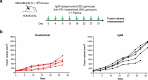
Inhibitors of programmed cell death protein 1 (PD-1) and its ligand (PD-L1) have dramatically changed the treatment landscape for patients with cancer. Clinical activity of anti-PD-(L)1 antibodies has resulted in increased median overall survival and durable responses in patients across selected tumor types. To date, 6 PD-1 and PD-L1, here collectively referred to as PD-(L)1, pathway inhibitors are approved by the US Food and Drug Administration for clinical use. The availability of multiple anti-PD-(L)1 antibodies provides treatment and dosing regimen choice for patients with cancer. Here, we describe the nonclinical characterization of dostarlimab (TSR-042), a humanized anti-PD-1 antibody, which binds with high affinity to human PD-1 and effectively inhibits its interaction with its ligands, PD-L1 and PD-L2. Dostarlimab enhanced effector T-cell functions, including cytokine production, in vitro. Since dostarlimab does not bind mouse PD-1, its single-agent antitumor activity was evaluated using humanized mouse models. In this model system, dostarlimab demonstrated antitumor activity as assessed by tumor growth inhibition, which was associated with increased infiltration of immune cells. Single-dose and 4-week repeat-dose toxicology studies in cynomolgus monkeys indicated that dostarlimab was well tolerated. In a clinical setting, based on data from the GARNET trial, dostarlimab (Jemperli) was approved for the treatment of adult patients with mismatch repair-deficient recurrent or advanced endometrial cancer that had progressed on or following prior treatment with a platinum-containing regimen. Taken together, these data demonstrate that dostarlimab is a potent anti-PD-1 receptor antagonist, with properties that support its continued clinical investigation in patients with cancer.
Keywords: Anti-PD-1 antibody; TSR-042; cancer; characterization; dostarlimab; immune checkpoint; solid tumors.
Conflict of interest statement
All authors are present or former employees of GlaxoSmithKline or AnaptysBio, Inc.
Figures




References
-
- US Food and Drug Administration . Opdivo (nivolumab) label. [accessed 2020 Sept 5]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125554s083lbl.pdf.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
