Dysbiotic microbiota interactions in Crohn's disease
- PMID: 34313550
- PMCID: PMC8320851
- DOI: 10.1080/19490976.2021.1949096
Dysbiotic microbiota interactions in Crohn's disease
Abstract
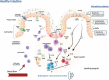
Crohn's disease (CD) is a major form of inflammatory bowel disease characterized by transmural inflammation along the alimentary tract. Changes in the microbial composition and reduction in species diversity are recognized as pivotal hallmarks in disease dynamics, challenging the gut barrier function and shaping a pathological immune response in genetically influenced subjects. The purpose of this review is to delve into the modification of the gut microbiota cluster network during CD progression and to discuss how this shift compromises the gut barrier integrity, granting the translocation of microbes and their products. We then complete the scope of the review by retracing gut microbiota dysbiosis interactions with the main pathophysiologic factors of CD, starting from the host's genetic background to the immune inflammatory and fibrotic processes, providing a standpoint on the lifestyle/exogenous factors and the potential benefits of targeting a specific gut microbiota.
Keywords: Crohn’s disease; bacterial translocation; dysbiosis; fibrosis; inflammation; microbiota.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
-
- Yilmaz B, Juillerat P, Øyås O, Ramon C, Bravo FD, Franc Y, Fournier N, Michetti P, Mueller C, Geuking M, et al. Microbial network disturbances in relapsing refractory Crohn’s disease. Nat Med. 2019;25(2):323–19. - PubMed
-
- Lavelle A, Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17(4):223–237. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
