Highly sensitive detection of antibody nonspecific interactions using flow cytometry
- PMID: 34313552
- PMCID: PMC8317921
- DOI: 10.1080/19420862.2021.1951426
Highly sensitive detection of antibody nonspecific interactions using flow cytometry
Abstract
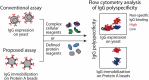
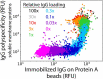
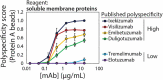
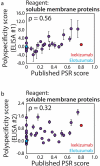
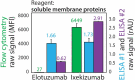
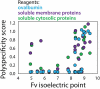
The rapidly evolving nature of antibody drug development has resulted in technologies that generate vast numbers (hundreds to thousands) of lead antibody candidates during early discovery. These candidates must be rapidly pared down to identify the most drug-like candidates for in-depth analysis of their safety and efficacy, which can only be performed on a limited number of antibodies due to time and resource requirements. One key biophysical property of successful antibody therapeutics is high specificity, defined as low levels of nonspecific binding or polyspecificity. Although there has been some progress in developing assays for detecting antibody polyspecificity, most of these assays are limited by poor sensitivity or assay formats that require proprietary antibody surface display methods, and some of these assays use complex and poorly defined polyspecificity reagents. Here we report the PolySpecificity Particle (PSP) assay, a sensitive flow cytometry assay for evaluating antibody nonspecific interactions that overcomes previous limitations and can be used for evaluating diverse types of IgGs, multispecific antibodies and Fc-fusion proteins. Our approach uses micron-sized magnetic beads coated with Protein A to capture antibodies at extremely dilute concentrations (<0.02 mg/mL). Flow cytometry analysis of polyspecificity reagent binding to these conjugates results in sensitive detection of differences in nonspecific interactions for clinical-stage antibodies. Our PSP assay strongly discriminates between antibodies with different levels of polyspecificity using previously reported polyspecificity reagents that are either well-defined proteins or highly complex protein mixtures. Moreover, we also find that a unique reagent, namely ovalbumin, results in the best assay sensitivity and specificity. Importantly, our assay is much more sensitive than standard assays such as ELISAs. We expect that our simple, sensitive, and high-throughput PSP assay will accelerate the development of safe and effective antibody therapeutics.
Keywords: Developability; affinity; bispecific; multispecific; mAb; IgG; Fc; Fab; non-specific; nonspecific; off-target binding; polyreactivity; specificity.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
-
- Dyson MR, Masters E, Pazeraitis D, Perera RL, Syrjanen JL, Surade S, Thorsteinson N, Parthiban K, Jones PC, Sattar M, et al. Beyond affinity: selection of antibody variants with optimal biophysical properties and reduced immunogenicity from mammalian display libraries. mAbs. 2020;12:1829335. doi: 10.1080/19420862.2020.1829335. - DOI - PMC - PubMed
-
- Shan L, Mody N, Sormani P, Rosenthal KL, Damschroder MM, Esfandiary R. Developability assessment of engineered monoclonal antibody variants with a complex self-association behavior using complementary analytical and in silico tools. Mol Pharm. 2018;15:5697–710. doi: 10.1021/acs.molpharmaceut.8b00867. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
