Effect of a Hospital and Postdischarge Quality Improvement Intervention on Clinical Outcomes and Quality of Care for Patients With Heart Failure With Reduced Ejection Fraction: The CONNECT-HF Randomized Clinical Trial
- PMID: 34313687
- PMCID: PMC8317015
- DOI: 10.1001/jama.2021.8844
Effect of a Hospital and Postdischarge Quality Improvement Intervention on Clinical Outcomes and Quality of Care for Patients With Heart Failure With Reduced Ejection Fraction: The CONNECT-HF Randomized Clinical Trial
Abstract
Importance: Adoption of guideline-directed medical therapy for patients with heart failure is variable. Interventions to improve guideline-directed medical therapy have failed to consistently achieve target metrics, and limited data exist to inform efforts to improve heart failure quality of care.
Objective: To evaluate the effect of a hospital and postdischarge quality improvement intervention compared with usual care on heart failure outcomes and care.
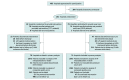
Design, setting, and participants: This cluster randomized clinical trial was conducted at 161 US hospitals and included 5647 patients (2675 intervention vs 2972 usual care) followed up after a hospital discharge for acute heart failure with reduced ejection fraction (HFrEF). The trial was performed from 2017 to 2020, and the date of final follow-up was August 31, 2020.
Interventions: Hospitals (n = 82) randomized to a hospital and postdischarge quality improvement intervention received regular education of clinicians by a trained group of heart failure and quality improvement experts and audit and feedback on heart failure process measures (eg, use of guideline-directed medical therapy for HFrEF) and outcomes. Hospitals (n = 79) randomized to usual care received access to a generalized heart failure education website.
Main outcomes and measures: The coprimary outcomes were a composite of first heart failure rehospitalization or all-cause mortality and change in an opportunity-based composite score for heart failure quality (percentage of recommendations followed).
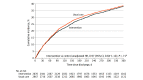
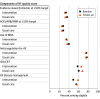
Results: Among 5647 patients (mean age, 63 years; 33% women; 38% Black; 87% chronic heart failure; 49% recent heart failure hospitalization), vital status was known for 5636 (99.8%). Heart failure rehospitalization or all-cause mortality occurred in 38.6% in the intervention group vs 39.2% in usual care (adjusted hazard ratio, 0.92 [95% CI, 0.81 to 1.05). The baseline quality-of-care score was 42.1% vs 45.5%, respectively, and the change from baseline to follow-up was 2.3% vs -1.0% (difference, 3.3% [95% CI, -0.8% to 7.3%]), with no significant difference between the 2 groups in the odds of achieving a higher composite quality score at last follow-up (adjusted odds ratio, 1.06 [95% CI, 0.93 to 1.21]).
Conclusions and relevance: Among patients with HFrEF in hospitals randomized to a hospital and postdischarge quality improvement intervention vs usual care, there was no significant difference in time to first heart failure rehospitalization or death, or in change in a composite heart failure quality-of-care score.
Trial registration: ClinicalTrials.gov Identifier: NCT03035474.
Conflict of interest statement
Figures
Comment in
-
Prioritizing Dissemination and Implementation Science in Cardiometabolic Medicine: CONNECTing the Dots.JAMA. 2021 Jul 27;326(4):311-313. doi: 10.1001/jama.2021.9847. JAMA. 2021. PMID: 34313706 No abstract available.
-
A Hospital and Postdischarge Quality Improvement Intervention and Outcomes and Care for Patients With Heart Failure With Reduced Ejection Fraction.JAMA. 2021 Nov 16;326(19):1977. doi: 10.1001/jama.2021.16753. JAMA. 2021. PMID: 34783843 No abstract available.
References
-
- Chan WV, Pearson TA, Bennett GC, et al. . ACC/AHA special report: clinical practice guideline implementation strategies: a summary of systematic reviews by the NHLBI Implementation Science Work Group: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;69(8):1076-1092. doi:10.1016/j.jacc.2016.11.004 - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

