Physician-patient communication affects patient satisfaction in treatment decision-making: a structural equation modelling analysis of a web-based survey in patients with ulcerative colitis
- PMID: 34313863
- PMCID: PMC8370900
- DOI: 10.1007/s00535-021-01811-1
Physician-patient communication affects patient satisfaction in treatment decision-making: a structural equation modelling analysis of a web-based survey in patients with ulcerative colitis
Abstract
Background: The relationship of bidirectional sharing of information between physicians and patients to patient satisfaction with treatment decision-making for ulcerative colitis (UC) has not been examined. Here, we conducted a web-based survey to evaluate this relationship.
Methods: Patients aged ≥ 20 years with UC were recruited from the IBD Patient Panel and Japanese IBD Patient Association. Patients completed our web-based survey between 11 May and 1 June 2020. The main outcomes were patient satisfaction (assessed by the Decision Regret Scale) and patient trust in physicians (assessed by the Trust in Physician Scale).
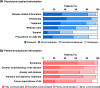
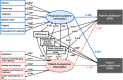
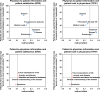
Results: In this study (n = 457), a structural equation modelling analysis showed that physician-to-patient and patient-to-physician information significantly affected patient satisfaction with treatment decision-making (standardised path coefficient: 0.426 and 0.135, respectively) and patient trust in physicians (0.587 and 0.158, respectively). Notably, physician-to-patient information had a greater impact. For patient satisfaction with treatment decision-making and patient trust in physicians, information on "disease" (indirect effect: 0.342 and 0.471, respectively), "treatment" (0.335 and 0.461, respectively), and "endoscopy" (0.295 and 0.407, respectively) was particularly important, and the level of this information was adequate or almost adequate. Patient-to-physician information on "anxiety and distress" (0.116 and 0.136, respectively), "intention and desire for treatment" (0.113 and 0.132, respectively), and "future expectations of life" (0.104 and 0.121, respectively) were also important for patient satisfaction with treatment decision-making and patient trust in physicians, but these concerns were not adequately communicated.
Conclusions: Adequate physician-patient communication, especially physician-to-patient information, enhanced patient satisfaction with treatment decision-making for UC.
Keywords: Patient satisfaction; Physician–patient communication; Ulcerative colitis.
© 2021. The Author(s).
Conflict of interest statement
KM has received grants and personal fees from AbbVie Inc., EA Pharma Co., Ltd., Kissei Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Mochida Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Pfizer Inc., Takeda Pharmaceutical Co., Ltd., and ZERIA Pharmaceutical Co., Ltd.; and personal fees from Janssen Pharmaceutical K.K., JIMRO Co., Ltd., and KYORIN Pharmaceutical Co., Ltd. HI has received personal fees from Janssen Pharmaceutical K.K. and Mitsubishi Tanabe Pharma Corporation. TN has received personal fees from Alexion Pharma GK., Baxter Ltd., Boehringer Ingelheim International GmbH, Chugai Pharmaceutical Co., Ltd., DENTSU Inc., Eli Lilly Japan K.K., Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Corporation, Nikkei Business Publications, Inc., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Pfizer Japan Inc., Sumitomo Dainippon Pharma Co., Ltd., and Takeda Pharmaceutical Co., Ltd.; and other remuneration from HANSHIN Dispensing Holding Co., Ltd., JMDC Inc., Nakagawa Pharmacy Co., Ltd., and Toyota Tsusho All Life Co. YH and AM have received personal fees from Janssen Pharmaceutical K.K. and Mitsubishi Tanabe Pharma Corporation. FH has received personal fees from AbbVie Inc., EA Pharma Co., Ltd., Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Corporation, Mochida Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Co., Ltd. FU has received personal fees from Takeda Pharmaceutical Co., Ltd. NS and YS are employees of Mitsubishi Tanabe Pharma Corporation. TH has received grants and personal fees from AbbVie Inc., JIMRO Co., Ltd., and ZERIA Pharmaceutical Co., Ltd.; grants from Otsuka Holdings Co., Ltd.; and personal fees from Aspen Japan K.K., Celltrion Healthcare Co., Ltd., EA Pharma Co., Ltd., Eli Lilly Japan K.K., Ferring Pharmaceuticals Co., Ltd., Gilead Sciences, Inc., Janssen Pharmaceutical K.K., KYORIN Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Mochida Pharmaceutical Co., Ltd., Nichi-Iko Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., and Takeda Pharmaceutical Co., Ltd.
Figures

References
-
- Ministry of Health Labour and Welfare research group (Suzuki group). Diagnostic criteria and treatment guidelines for ulcerative colitis and Crohn’s disease. Supplemental volume, Annual report. 2020. http://www.ibdjapan.org/pdf/doc01.pdf. Accessed 20 Jan 2021 (in Japanese)
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

