Aging-Induced Impairment of Vascular Function: Mitochondrial Redox Contributions and Physiological/Clinical Implications
- PMID: 34314229
- PMCID: PMC8905248
- DOI: 10.1089/ars.2021.0031
Aging-Induced Impairment of Vascular Function: Mitochondrial Redox Contributions and Physiological/Clinical Implications
Abstract
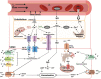
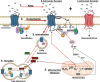
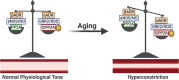
Significance: The vasculature responds to the respiratory needs of tissue by modulating luminal diameter through smooth muscle constriction or relaxation. Coronary perfusion, diastolic function, and coronary flow reserve are drastically reduced with aging. This loss of blood flow contributes to and exacerbates pathological processes such as angina pectoris, atherosclerosis, and coronary artery and microvascular disease. Recent Advances: Increased attention has recently been given to defining mechanisms behind aging-mediated loss of vascular function and development of therapeutic strategies to restore youthful vascular responsiveness. The ultimate goal aims at providing new avenues for symptom management, reversal of tissue damage, and preventing or delaying of aging-induced vascular damage and dysfunction in the first place. Critical Issues: Our major objective is to describe how aging-associated mitochondrial dysfunction contributes to endothelial and smooth muscle dysfunction via dysregulated reactive oxygen species production, the clinical impact of this phenomenon, and to discuss emerging therapeutic strategies. Pathological changes in regulation of mitochondrial oxidative and nitrosative balance (Section 1) and mitochondrial dynamics of fission/fusion (Section 2) have widespread effects on the mechanisms underlying the ability of the vasculature to relax, leading to hyperconstriction with aging. We will focus on flow-mediated dilation, endothelial hyperpolarizing factors (Sections 3 and 4), and adrenergic receptors (Section 5), as outlined in Figure 1. The clinical implications of these changes on major adverse cardiac events and mortality are described (Section 6). Future Directions: We discuss antioxidative therapeutic strategies currently in development to restore mitochondrial redox homeostasis and subsequently vascular function and evaluate their potential clinical impact (Section 7). Antioxid. Redox Signal. 35, 974-1015.
Keywords: aging; endothelial dysfunction; mitochondrial dysfunction; oxidative stress; reactive oxygen species; vasodilation.
Conflict of interest statement
No competing financial interests exist.
Figures


References
-
- Accardi G, Aiello A, Gambino CM, Virruso C, Caruso C, and Candore G. Mediterranean nutraceutical foods: strategy to improve vascular ageing. Mech Ageing Dev 159: 63–70, 2016. - PubMed
-
- Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, Wakisaka M, Ibayashi S, Utsumi H, and Iida M. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation 109: 227–233, 2004. - PubMed
-
- Ahn S, Maudsley S, Luttrell LM, Lefkowitz RJ, and Daaka Y. Src-mediated tyrosine phosphorylation of dynamin is required for beta2-adrenergic receptor internalization and mitogen-activated protein kinase signaling. J Biol Chem 274: 1185–1188, 1999. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

