The spatiotemporal organization of experience dictates hippocampal involvement in primary visual cortical plasticity
- PMID: 34314678
- PMCID: PMC8524775
- DOI: 10.1016/j.cub.2021.06.079
The spatiotemporal organization of experience dictates hippocampal involvement in primary visual cortical plasticity
Abstract
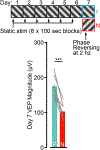
The hippocampus and neocortex are theorized to be crucial partners in the formation of long-term memories. Here, we assess hippocampal involvement in two related forms of experience-dependent plasticity in the primary visual cortex (V1) of mice. Like control animals, those with hippocampal lesions exhibit potentiation of visually evoked potentials after passive daily exposure to a phase-reversing oriented grating stimulus, which is accompanied by long-term habituation of a reflexive behavioral response. Thus, low-level recognition memory is formed independently of the hippocampus. However, response potentiation resulting from daily exposure to a fixed sequence of four oriented gratings is severely impaired in mice with hippocampal damage. A feature of sequence plasticity in V1 of controls, which is absent in lesioned mice, is the generation of predictive responses to an anticipated stimulus element when it is withheld or delayed. Thus, the hippocampus is involved in encoding temporally structured experience, even within the primary sensory cortex.
Keywords: hippocampus; long-term memory; primary visual cortex; synaptic plasticity; systems consolidation.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests. R.W.K. is currently an employee of Biogen, Inc.
Figures



Comment in
-
Visual plasticity: Illuminating the role of the hippocampus in cortical sensory encoding.Curr Biol. 2021 Sep 27;31(18):R1087-R1089. doi: 10.1016/j.cub.2021.07.015. Curr Biol. 2021. PMID: 34582817
References
-
- Squire LR (1992). Declarative and nondeclarative memory: multiple brain systems supporting learning and memory. J. Cogn. Neurosci 4, 232–243. - PubMed
-
- Nissen MJ, Knopman DS & Schacter DL (1987). Neurochemical dissociation of memory systems. Neurology 37, 789–794. - PubMed
-
- Squire LR (2004). Memory systems of the brain: a brief history and current perspective. Neurobiol. learn. and mem 82, 171–177. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

