Ribosome ADP-ribosylation inhibits translation and maintains proteostasis in cancers
- PMID: 34314702
- PMCID: PMC8380725
- DOI: 10.1016/j.cell.2021.07.005
Ribosome ADP-ribosylation inhibits translation and maintains proteostasis in cancers
Abstract
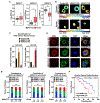
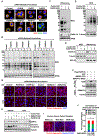
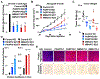
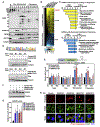
Defects in translation lead to changes in the expression of proteins that can serve as drivers of cancer formation. Here, we show that cytosolic NAD+ synthesis plays an essential role in ovarian cancer by regulating translation and maintaining protein homeostasis. Expression of NMNAT-2, a cytosolic NAD+ synthase, is highly upregulated in ovarian cancers. NMNAT-2 supports the catalytic activity of the mono(ADP-ribosyl) transferase (MART) PARP-16, which mono(ADP-ribosyl)ates (MARylates) ribosomal proteins. Depletion of NMNAT-2 or PARP-16 leads to inhibition of MARylation, increased polysome association and enhanced translation of specific mRNAs, aggregation of their translated protein products, and reduced growth of ovarian cancer cells. Furthermore, MARylation of the ribosomal proteins, such as RPL24 and RPS6, inhibits polysome assembly by stabilizing eIF6 binding to ribosomes. Collectively, our results demonstrate that ribosome MARylation promotes protein homeostasis in cancers by fine-tuning the levels of protein synthesis and preventing toxic protein aggregation.
Keywords: ADP-ribosylation; MARylation; NAD(+); NAD(+) sensor; NAD(+) synthesis; NMNAT-2; PARP-16; cancer; mRNA translation; mono(ADP-ribose); mono(ADP-ribosyl)ation; ovarian cancer; protein aggregation; protein synthesis; ribosomes; translation.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests W.L.K. is a founder and consultant for Ribon Therapeutics, Inc. and ARase Therapeutics, Inc. He is also coholder of U.S. Patent 9,599,606 covering the ADP-ribose detection reagent used herein, which has been licensed to and is sold by EMD Millipore.
Figures


References
-
- Andrews S (2010). FASTQC. A quality control tool for high throughput sequence data.
-
- Berger F, Lau C, Dahlmann M, and Ziegler M (2005). Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J Biol Chem 280, 36334–36341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

