Therapeutic targets, novel drugs, and delivery systems for diabetes associated NAFLD and liver fibrosis
- PMID: 34314787
- PMCID: PMC8440458
- DOI: 10.1016/j.addr.2021.113888
Therapeutic targets, novel drugs, and delivery systems for diabetes associated NAFLD and liver fibrosis
Abstract
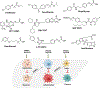
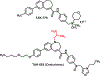
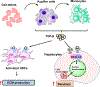
Type 2 diabetes mellitus (T2DM) associated non-alcoholic fatty liver disease (NAFLD) is the fourth-leading cause of death. Hyperglycemia induces various complications, including nephropathy, cirrhosis and eventually hepatocellular carcinoma (HCC). There are several etiological factors leading to liver disease development, which involve insulin resistance and oxidative stress. Free fatty acid (FFA) accumulation in the liver exerts oxidative and endoplasmic reticulum (ER) stresses. Hepatocyte injury induces release of inflammatory cytokines from Kupffer cells (KCs), which are responsible for activating hepatic stellate cells (HSCs). In this review, we will discuss various molecular targets for treating chronic liver diseases, including homeostasis of FFA, lipid metabolism, and decrease in hepatocyte apoptosis, role of growth factors, and regulation of epithelial-to-mesenchymal transition (EMT) and HSC activation. This review will also critically assess different strategies to enhance drug delivery to different cell types. Targeting nanocarriers to specific liver cell types have the potential to increase efficacy and suppress off-target effects.
Keywords: Cirrhosis; Diabetes; Hepatocellular carcinoma; Inflammation; Liver fibrosis; NAFLD.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
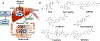
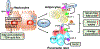
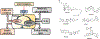


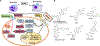

References
-
- Centers for Disease Control and Prevention, Chronic Liver Disease and Cirrhosis, CDC 2021 (2021) .
-
- Ren Z, Li A, Jiang J, Zhou L, Yu Z, Lu H, Xie H, Chen X, Shao L, Zhang R, Xu S, Zhang H, Cui G, Chen X, Sun R, Wen H, Lerut JP, Kan Q, Li L, Zheng S, Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma, Gut 68 (2019) 1014–1023. - PMC - PubMed
-
- Makri E, Goulas A, Polyzos SA, Epidemiology, Pathogenesis, Diagnosis and Emerging Treatment of Nonalcoholic Fatty Liver Disease, Arch. Med. Res 52 (2021) 25–37. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

