Patient-Reported Outcomes and Patient-Reported Experience of Patients With Atrial Fibrillation in the IMPACT-AF Clinical Trial
- PMID: 34315232
- PMCID: PMC8475702
- DOI: 10.1161/JAHA.120.019783
Patient-Reported Outcomes and Patient-Reported Experience of Patients With Atrial Fibrillation in the IMPACT-AF Clinical Trial
Abstract
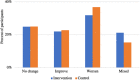
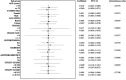
Background The IMPACT-AF (Integrated Management Program Advancing Community Treatment of Atrial Fibrillation) trial is a prospective, randomized, cluster design trial comparing atrial fibrillation management with a computerized clinical decision support system with usual care (control) in the primary care setting of Nova Scotia, Canada. The objective of this analysis was to assess and compare patient-reported health-related quality of life and patient-reported experience with atrial fibrillation care between clinical decision support and control groups. Methods and Results Health-related quality of life was measured using the EuroQol 5-dimensional 5-level scale, whereas patient-reported experience was assessed using a self-administered satisfaction questionnaire, both assessed at baseline and 12 months. Health utilities were calculated using the Canadian EuroQol 5-dimensional 5-level value set. Descriptive statistics and generalized estimating equations were used to compare between groups. Among 1145 patients enrolled in the trial, 717 had complete EuroQol 5-dimensional 5-level data at baseline. The mean age of patients was 73.53 years, and 61.87% were men. Mean utilities at baseline were 0.809 (SD, 0.157) and 0.814 (SD, 0.157) for clinical decision support and control groups, respectively. At baseline, most patients in both groups reported being "very satisfied" with the care received for their atrial fibrillation. There were no statistically significant differences in utility scores or patient satisfaction between groups at 12 months. Conclusions Health-related quality of life of patients remained stable over 12 months, and there was no significant difference in patient satisfaction or utility scores between clinical decision support and control groups. Registration information clinicaltrials.gov. Identifier: NCT01927367.
Keywords: atrial fibrillation; clinical trial; health utility; health‐related quality of life; patient satisfaction.
Conflict of interest statement
B. Humphries has a Doctoral Award from the Canadian Institutes of Health Research. Dr Cox reports grants from Bayer Inc during the conduct of the study; and personal fees from Bayer and Servier, outside the submitted work. Dr Parkash reports grants from Bayer and Pfizer, during the conduct of the study. Dr MacKillop reports other fees from Merck Canada, Bayer, and Pfizer, outside the submitted work. A. Ciaccia and Dr Choudhri are employees of Bayer Inc. J. Nemis‐White reports personal fees from Nova Scotia Health Authority during the conduct of the study. The remaining authors have no disclosures to report.
Figures


References
-
- Heart & Stroke . Atrial fibrillation. 2020. (cited June 25, 2020). https://www.heartandstroke.ca/heart/conditions/atrial‐fibrillation. Accessed June 12, 2020.
-
- Witassek F, Springer A, Adam L, Aeschbacher S, Beer JH, Blum S, Bonati LH, Conen D, Kobza R, Kühne M, et al. Health‐related quality of life in patients with atrial fibrillation: the role of symptoms, comorbidities, and the type of atrial fibrillation. PLoS One. 2019;14:e0226730. DOI: 10.1371/journal.pone.0226730. - DOI - PMC - PubMed
-
- January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. Circulation. 2014;130:2071–2104. DOI: 10.1161/CIR.0000000000000040. - DOI - PubMed
-
- Grönefeld G, Hohnloser S. Quality of life in atrial fibrillation: an increasingly important issue. Eur Heart J. 2003;5:H25–H33. DOI: 10.1016/S1520-765X(03)90020-4. - DOI
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

