Extracellular volume-guided late gadolinium enhancement analysis for non-ischemic cardiomyopathy: The Women's Interagency HIV Study
- PMID: 34315432
- PMCID: PMC8314536
- DOI: 10.1186/s12880-021-00649-6
Extracellular volume-guided late gadolinium enhancement analysis for non-ischemic cardiomyopathy: The Women's Interagency HIV Study
Abstract
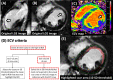
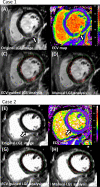
Background: Quantification of non-ischemic myocardial scar remains a challenge due to the patchy diffuse nature of fibrosis. Extracellular volume (ECV) to guide late gadolinium enhancement (LGE) analysis may achieve a robust scar assessment.
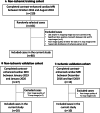
Methods: Three cohorts of 80 non-ischemic-training, 20 non-ischemic-validation, and 10 ischemic-validation were prospectively enrolled and underwent 3.0 Tesla cardiac MRI. An ECV cutoff to differentiate LGE scar from non-scar was identified in the training cohort from the receiver-operating characteristic curve analysis, by comparing the ECV value against the visually-determined presence/absence of the LGE scar at the highest signal intensity (SI) area of the mid-left ventricle (LV) LGE. Based on the ECV cutoff, an LGE semi-automatic threshold of n-times of standard-deviation (n-SD) above the remote-myocardium SI was optimized in the individual cases ensuring correspondence between LGE and ECV images. The inter-method agreement of scar amount in comparison with manual (for non-ischemic) or full-width half-maximum (FWHM, for ischemic) was assessed. Intra- and inter-observer reproducibility were investigated in a randomly chosen subset of 40 non-ischemic and 10 ischemic cases.
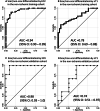
Results: The non-ischemic groups were all female with the HIV positive rate of 73.8% (training) and 80% (validation). The ischemic group was all male with reduced LV function. An ECV cutoff of 31.5% achieved optimum performance (sensitivity: 90%, specificity: 86.7% in training; sensitivity: 100%, specificity: 81.8% in validation dataset). The identified n-SD threshold varied widely (range 3 SD-18 SD), and was independent of scar amount (β = -0.01, p = 0.92). In the non-ischemic cohorts, results suggested that the manual LGE assessment overestimated scar (%) in comparison to ECV-guided analysis [training: 4.5 (3.2-6.4) vs. 0.92 (0.1-2.1); validation: 2.5 (1.2-3.7) vs. 0.2 (0-1.6); P < 0.01 for both]. Intra- and inter-observer analyses of global scar (%) showed higher reproducibility in ECV-guided than manual analysis with CCC = 0.94 and 0.78 versus CCC = 0.86 and 0.73, respectively (P < 0.01 for all). In ischemic validation, the ECV-guided LGE analysis showed a comparable scar amount and reproducibility with the FWHM.
Conclusions: ECV-guided LGE analysis is a robust scar quantification method for a non-ischemic cohort. Trial registration ClinicalTrials.gov; NCT00000797, retrospectively-registered 2 November 1999; NCT02501811, registered 15 July 2015.
Keywords: ECV-guided LGE analysis; Extracellular volume fraction (ECV); Human immunodeficiency virus (HIV); Late gadolinium enhancement (LGE); Magnetic resonance imaging (MRI); Non-ischemic LGE; Scar quantification.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Chan RH, Maron BJ, Olivotto I, Pencina MJ, Assenza GE, Haas T, et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation. 2014;130:484–495. doi: 10.1161/CIRCULATIONAHA.113.007094. - DOI - PubMed
-
- Youn J-C, Hong YJ, Lee H-J, Han K, Shim CY, Hong G-R, et al. Contrast-enhanced T1 mapping-based extracellular volume fraction independently predicts clinical outcome in patients with non-ischemic dilated cardiomyopathy: a prospective cohort study. Eur Radiol. 2017;27:3924–3933. doi: 10.1007/s00330-017-4817-9. - DOI - PubMed
-
- Gräni C, Eichhorn C, Bière L, Kaneko K, Murthy VL, Agarwal V, et al. Comparison of myocardial fibrosis quantification methods by cardiovascular magnetic resonance imaging for risk stratification of patients with suspected myocarditis. J Cardiovasc Magn Reson. 2019;21:1–11. doi: 10.1186/s12968-019-0520-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

