Evaluation of the safety profile of COVID-19 vaccines: a rapid review
- PMID: 34315454
- PMCID: PMC8315897
- DOI: 10.1186/s12916-021-02059-5
Evaluation of the safety profile of COVID-19 vaccines: a rapid review
Abstract
Background: The rapid process of research and development and lack of follow-up time post-vaccination aroused great public concern about the safety profile of COVID-19 vaccine candidates. To provide comprehensive overview of the safety profile of COVID-19 vaccines by using meta-analysis technique.
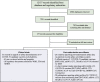
Methods: English-language articles and results posted on PubMed, Embase, Web of Science, PMC, official regulatory websites, and post-authorization safety surveillance data were searched through June 12, 2021. Publications disclosing safety data of COVID-19 candidate vaccines in humans were included. A meta-analysis of proportions was performed to estimate the pooled incidence and the pooled rate ratio (RR) of safety outcomes of COVID-19 vaccines using different platforms.
Results: A total of 87 publications with safety data from clinical trials and post-authorization studies of 19 COVID-19 vaccines on 6 different platforms were included. The pooled rates of local and systemic reactions were significantly lower among inactivated vaccines (23.7%, 21.0%), protein subunit vaccines (33.0%, 22.3%), and DNA vaccines (39.5%, 29.3%), compared to RNA vaccines (89.4%, 83.3%), non-replicating vector vaccines (55.9%, 66.3%), and virus-like particle vaccines (100.0%, 78.9%). Solicited injection-site pain was the most common local reactions, and fatigue and headache were the most common systemic reactions. The frequency of vaccine-related serious adverse events was low (< 0.1%) and balanced between treatment groups. Vaccine platforms and age groups of vaccine recipients accounted for much of the heterogeneity in safety profiles between COVID-19 vaccines. Reporting rates of adverse events from post-authorization observational studies were similar to results from clinical trials. Crude reporting rates of adverse events from post-authorization safety monitoring (passive surveillance) were lower than in clinical trials and varied between countries.
Conclusions: Available evidence indicates that eligible COVID-19 vaccines have an acceptable short-term safety profile. Additional studies and long-term population-level surveillance are strongly encouraged to further define the safety profile of COVID-19 vaccines.
Keywords: Novel coronavirus diseases 2019; Review; Safety profile; Severe acute respiratory syndrome coronavirus 2; Vaccine.
© 2021. The Author(s).
Conflict of interest statement
H.Y. has received research funding from Sanofi Pasteur, GlaxoSmithKline, Yichang HEC Changjiang Pharmaceutical Company, and Shanghai Roche Pharmaceutical Company. M.D. has received research support from Walgreen Company and Merck. D.S. has received consulting or grant funding from Merck and Janssen. None of those research funding is related to development of COVID-19 vaccines. All other authors report no competing interests.
Figures


References
-
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus Investigating and Research Team A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. - DOI - PMC - PubMed
-
- World Health Organization situation report. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio.... Accessed 8 May 2021.
-
- Draft landscape and tracker of COVID-19 candidate vaccines. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand.... Accessed 11 May 2021.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

