JunD accentuates arecoline-induced disruption of tight junctions and promotes epithelial-to-mesenchymal transition by association with NEAT1 lncRNA
- PMID: 34316331
- PMCID: PMC8310672
- DOI: 10.18632/oncotarget.28026
JunD accentuates arecoline-induced disruption of tight junctions and promotes epithelial-to-mesenchymal transition by association with NEAT1 lncRNA
Abstract
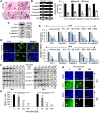
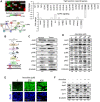
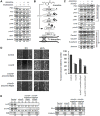
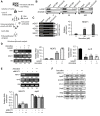
Head and neck cancers are highly prevalent in south-east Asia, primarily due to betel nut chewing. Arecoline, the primary alkaloid is highly carcinogenic; however its role in promoting tumorigenesis by disrupting junctional complexes and increasing risk of metastasis is not well delineated. Subsequently, the effects of low and high concentrations of arecoline on the stability of tight junctions and EMT induction were studied. A microarray analysis confirmed involvement of a MAPK component, JunD, in regulating tight junction-associated genes, specifically ZO-1. Results established that although arecoline-induced phosphorylation of JunD downregulated expression of ZO-1, JunD itself was modulated by the lncRNA-NEAT1 in presence of arecoline. Increased NEAT1 in tissues of HNSCC patients significantly correlated with poor disease prognosis. Here we show that NEAT1-JunD complex interacted with ZO-1 promoter in the nuclear compartment, downregulated expression of ZO-1 and destabilized tight junction assembly. Consequently, silencing NEAT1 in arecoline-exposed cells not only downregulated the expression of JunD and stabilized expression of ZO-1, but also reduced expression of the EMT markers, Slug and Snail, indicating its direct regulatory role in arecoline-mediated TJ disruption and disease progression.
Keywords: JunD; arecoline; head and neck cancer; lncRNA-NEAT1; tight junction.
Copyright: © 2021 Ghosh et al.
Conflict of interest statement
CONFLICTS OF INTEREST Authors have no conflicts of interest to declare.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials

