Phenolic profile of a Parma violet unveiled by chemical and fluorescence imaging
- PMID: 34316339
- PMCID: PMC8300547
- DOI: 10.1093/aobpla/plab041
Phenolic profile of a Parma violet unveiled by chemical and fluorescence imaging
Abstract
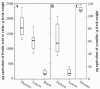
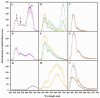
The ability of phenolic compounds to autofluoresce upon illumination by UV or blue light was exploited to explore the nature and distribution of these metabolites within the flower petals, leaves and roots of the violet, Viola alba subsp. dehnhardtii. This was achieved through a dual complementary approach that combined fluorescence microscopy imaging of living intact tissues and chemical extraction of pulverized material. The blue to red fluorescence displayed by living tissues upon illumination was indicative of their richness in phenolic compounds. Phenolic acids were found in all tissues, while flavonoids characterized the aerial part of the plant, anthocyanidins being restricted to the petals. The chemical quantification of phenolics in plant extracts confirmed their tissue-specific distribution and abundance. A key finding was that the spectral signatures obtained through confocal microscopy of endogenous fluorophores in living tissues and their counterpart extracts share the same fluorescence patterns, pointing out the potential of fluorescence imaging of intact organs for a proper estimation of their phenolic content. In addition, this study highlighted a few distinct morphology cell types, in particular foliar-glandular-like structures, and jagged petal cell walls. Altogether, these data provide a comprehensive histochemical localization of phenolics in living tissues of a violet. Converting fluorescence imaging into a chemical imprint indicated that one can rely on fluorescence microscopy of intact living tissues as a rapid, non-destructive means to follow their phenolic imprint under various environmental conditions.
Keywords: Autofluorescence; Parma violet; Viola alba subsp. dehnhardtii; chemical quantification; confocal microscopy; phenolic profile.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Annals of Botany Company.
Figures


References
-
- Bahorun T, Trotina F, Vasseurt J. 1994. Comparative polyphenolic productions in Crataegus monogyna callus cultures. Phytochemistry 37:1273–1276.
-
- Benvenuti S, Bortolotti E, Maggin R. 2016. Antioxidant power, anthocyanin content and organoleptic performance of edible flowers. Scientia Horticulturae 199:170–177.
-
- Buschmann C, Langsdorf G, Lichtenthaler HK. 2000. Imaging of the blue, green and red fluorescence emission of plants. Photosynthetica 38:483–491.
-
- Chervin J, Perio P, Martins-Froment N. 2017. Dereplication of natural products from complex extracts by regression analysis and molecular networking: case study of redox-active compounds from Viola alba subsp. dehnhardtii. Metabolomics 13:96.
-
- Chervin J, Talou T, Audonnet M. 2019. Deciphering the phylogeny of violets based on multiplexed genetic and metabolomic approaches. Phytochemistry 163:99–110. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

