Therapeutic management of patients with COVID-19: a systematic review
- PMID: 34316558
- PMCID: PMC7162768
- DOI: 10.1016/j.infpip.2020.100061
Therapeutic management of patients with COVID-19: a systematic review
Abstract
Background: Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is the causative agent of coronavirus disease 2019 (COVID-19), which was declared a global pandemic by the World Health Organization on 11th March 2020. The treatment guidelines for COVID-19 vary between countries, yet there is no approved treatment to date.
Aim: To report any evidence of therapeutics used for the management of patients with COVID-19 in clinical practice since emergence of the virus.
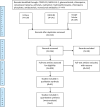
Methods: A systematic review protocol was developed based on the PRISMA statement. Articles for review were selected from Embase, Medline and Google Scholar. Readily accessible peer-reviewed, full articles in English published from 1st December 2019 to 26th March 2020 were included. The search terms included combinations of: COVID, SARS-COV-2, glucocorticoids, convalescent plasma, antiviral and antibacterial. There were no restrictions on the types of study eligible for inclusion.
Results: Four hundred and forty-nine articles were identified in the literature search; of these, 41 studies were included in this review. These were clinical trials (N=3), case reports (N=7), case series (N=10), and retrospective (N=11) and prospective (N=10) observational studies. Thirty-six studies were conducted in China (88%). Corticosteroid treatment was reported most frequently (N=25), followed by lopinavir (N=21) and oseltamivir (N=16).
Conclusions: This is the first systematic review to date related to medication used to treat patients with COVID-19. Only 41 studies were eligible for inclusion, most of which were conducted in China. Corticosteroid treatment was reported most frequently in the literature.
Keywords: Arbidol hydrochloride; COVID-19; Convalescent plasma therapy; Corticosteroids; Hydroxychloroquine; SARS-CoV-2.
© 2020 The Authors.
Figures
References
-
- Yang W., Cao Q., Qin L., Wang X., Cheng Z., Pan A. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020;S0163–4453(20):388–393. doi: 10.1016/j.jinf.2020.02.016. [published online ahead of print, 2020 Feb 26] - DOI - PMC - PubMed
-
- Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Ther. 2020;14:58–60. - PubMed
-
- Chen C., Qi F., Shi K., Li Y., Li J., Chen Y. Thalidomide combined with low-dose glucocorticoid in the treatment of COVID-19 pneumonia. Preprints. 2020:2020020395. https://www.preprints.org/manuscript/202002.0395/v1
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous