Osimertinib-Induced Cardiomyopathy
- PMID: 34317311
- PMCID: PMC8298525
- DOI: 10.1016/j.jaccas.2019.12.038
Osimertinib-Induced Cardiomyopathy
Abstract
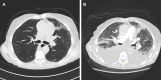
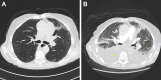
Osimertinib is the preferred treatment in patients with metastatic non-small cell lung cancer with epidermal growth factor receptor mutations. We report a case series of acute cardiomyopathy with heart failure exacerbation during osimertinib treatment. We suggest that cardiotoxicity from osimertinib is reversible and occurs at a dose of 80 mg/day. (Level of Difficulty: Intermediate.).
Keywords: CHF, congestive heart failure; EF, ejection fraction; EGFR, epidermal growth factor receptor; NSCLC, non–small cell lung cancer; TKI, tyrosine kinase inhibitor; cardio-oncology; cardiotoxicity; heart failure; osimertinib; reduced ejection fraction.
© 2020 The Authors.
Figures
References
-
- Soria J.-C., Ohe Y., Vansteenkiste J. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378:113–125. - PubMed
-
- Goss G., Tsai C.M., Shepherd F.A. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2016;17:1643–1652. - PubMed
-
- U.S. Food and Drug Administration Center for Drug Evaluation and Research. TAGRISSO (osimertinib) tablets, for oral use. http://www.fda.gov/medwatch Available at:
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous