Phase I study in healthy participants to evaluate safety, tolerability, and pharmacokinetics of inhaled nezulcitinib, a potential treatment for COVID-19
- PMID: 34318597
- PMCID: PMC8444931
- DOI: 10.1111/cts.13123
Phase I study in healthy participants to evaluate safety, tolerability, and pharmacokinetics of inhaled nezulcitinib, a potential treatment for COVID-19
Abstract
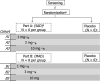
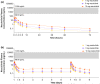
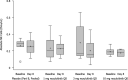
Nezulcitinib (TD-0903), a lung-selective pan-Janus-associated kinase (JAK) inhibitor designed for inhaled delivery, is under development for treatment of acute lung injury associated with coronavirus disease 2019 (COVID-19). This two-part, double-blind, randomized, placebo-controlled, single ascending dose (part A) and multiple ascending dose (part B) phase I study evaluated the safety, tolerability, and pharmacokinetics (PK) of nezulcitinib in healthy participants. Part A included three cohorts randomized 6:2 to receive a single inhaled dose of nezulcitinib (1, 3, or 10 mg) or matching placebo. Part B included three cohorts randomized 8:2 to receive inhaled nezulcitinib (1, 3, or 10 mg) or matching placebo for 7 days. The primary outcome was nezulcitinib safety and tolerability assessed from treatment-emergent adverse events (TEAEs). The secondary outcome was nezulcitinib PK. All participants completed the study. All TEAEs were mild or moderate in severity, and none led to treatment discontinuation. Overall (area under the plasma concentration-time curve) and peak (maximal plasma concentration) plasma exposures of nezulcitinib were low and increased in a dose-proportional manner from 1 to 10 mg in both parts, with no suggestion of clinically meaningful drug accumulation. Maximal plasma exposures were below levels expected to result in systemic target engagement, consistent with a lung-selective profile. No reductions in natural killer cell counts were observed, consistent with the lack of a systemic pharmacological effect and the observed PK. In summary, single and multiple doses of inhaled nezulcitinib at 1, 3, and 10 mg were well-tolerated in healthy participants, with dose-proportional PK supporting once-daily administration.
© 2021 Theravance Biopharma R&D IP, LLC. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of the American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
N.D.P., A.L., D.L.B., and K.C. are employees of Theravance Biopharma US, Inc.; and shareholders in Theravance Biopharma, Inc. D.S. reports personal fees from Theravance Biopharma Ireland Limited during the conduct of the study; and personal fees from AstraZeneca; Boehringer Ingelheim; Chiesi; Cipla; Genentech; GlaxoSmithKline; Glenmark; Menarini; Mundipharma; Novartis; Peptinnovate; Pfizer; Pulmatrix; Theravance Biopharma Ireland Limited; and Verona outside this work.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

