Ivermectin for preventing and treating COVID-19
- PMID: 34318930
- PMCID: PMC8406455
- DOI: 10.1002/14651858.CD015017.pub2
Ivermectin for preventing and treating COVID-19
Update in
-
Ivermectin for preventing and treating COVID-19.Cochrane Database Syst Rev. 2022 Jun 21;6(6):CD015017. doi: 10.1002/14651858.CD015017.pub3. Cochrane Database Syst Rev. 2022. PMID: 35726131 Free PMC article.
Abstract
Background: Ivermectin, an antiparasitic agent used to treat parasitic infestations, inhibits the replication of viruses in vitro. The molecular hypothesis of ivermectin's antiviral mode of action suggests an inhibitory effect on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) replication in the early stages of infection. Currently, evidence on efficacy and safety of ivermectin for prevention of SARS-CoV-2 infection and COVID-19 treatment is conflicting.
Objectives: To assess the efficacy and safety of ivermectin compared to no treatment, standard of care, placebo, or any other proven intervention for people with COVID-19 receiving treatment as inpatients or outpatients, and for prevention of an infection with SARS-CoV-2 (postexposure prophylaxis).
Search methods: We searched the Cochrane COVID-19 Study Register, Web of Science (Emerging Citation Index and Science Citation Index), medRxiv, and Research Square, identifying completed and ongoing studies without language restrictions to 26 May 2021.
Selection criteria: We included randomized controlled trials (RCTs) comparing ivermectin to no treatment, standard of care, placebo, or another proven intervention for treatment of people with confirmed COVID-19 diagnosis, irrespective of disease severity, treated in inpatient or outpatient settings, and for prevention of SARS-CoV-2 infection. Co-interventions had to be the same in both study arms. We excluded studies comparing ivermectin to other pharmacological interventions with unproven efficacy.
Data collection and analysis: We assessed RCTs for bias, using the Cochrane risk of bias 2 tool. The primary analysis excluded studies with high risk of bias. We used GRADE to rate the certainty of evidence for the following outcomes 1. to treat inpatients with moderate-to-severe COVID-19: mortality, clinical worsening or improvement, adverse events, quality of life, duration of hospitalization, and viral clearance; 2. to treat outpatients with mild COVID-19: mortality, clinical worsening or improvement, admission to hospital, adverse events, quality of life, and viral clearance; (3) to prevent SARS-CoV-2 infection: SARS-CoV-2 infection, development of COVID-19 symptoms, adverse events, mortality, admission to hospital, and quality of life.
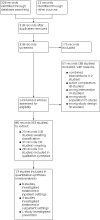
Main results: We found 14 studies with 1678 participants investigating ivermectin compared to no treatment, placebo, or standard of care. No study compared ivermectin to an intervention with proven efficacy. There were nine studies treating participants with moderate COVID-19 in inpatient settings and four treating mild COVID-19 cases in outpatient settings. One study investigated ivermectin for prevention of SARS-CoV-2 infection. Eight studies had an open-label design, six were double-blind and placebo-controlled. Of the 41 study results contributed by included studies, about one third were at overall high risk of bias. Ivermectin doses and treatment duration varied among included studies. We identified 31 ongoing and 18 studies awaiting classification until publication of results or clarification of inconsistencies. Ivermectin compared to placebo or standard of care for inpatient COVID-19 treatment We are uncertain whether ivermectin compared to placebo or standard of care reduces or increases mortality (risk ratio (RR) 0.60, 95% confidence interval (CI) 0.14 to 2.51; 2 studies, 185 participants; very low-certainty evidence) and clinical worsening up to day 28 assessed as need for invasive mechanical ventilation (IMV) (RR 0.55, 95% CI 0.11 to 2.59; 2 studies, 185 participants; very low-certainty evidence) or need for supplemental oxygen (0 participants required supplemental oxygen; 1 study, 45 participants; very low-certainty evidence), adverse events within 28 days (RR 1.21, 95% CI 0.50 to 2.97; 1 study, 152 participants; very low-certainty evidence), and viral clearance at day seven (RR 1.82, 95% CI 0.51 to 6.48; 2 studies, 159 participants; very low-certainty evidence). Ivermectin may have little or no effect compared to placebo or standard of care on clinical improvement up to 28 days (RR 1.03, 95% CI 0.78 to 1.35; 1 study; 73 participants; low-certainty evidence) and duration of hospitalization (mean difference (MD) -0.10 days, 95% CI -2.43 to 2.23; 1 study; 45 participants; low-certainty evidence). No study reported quality of life up to 28 days. Ivermectin compared to placebo or standard of care for outpatient COVID-19 treatment We are uncertain whether ivermectin compared to placebo or standard of care reduces or increases mortality up to 28 days (RR 0.33, 95% CI 0.01 to 8.05; 2 studies, 422 participants; very low-certainty evidence) and clinical worsening up to 14 days assessed as need for IMV (RR 2.97, 95% CI 0.12 to 72.47; 1 study, 398 participants; very low-certainty evidence) or non-IMV or high flow oxygen requirement (0 participants required non-IMV or high flow; 1 study, 398 participants; very low-certainty evidence). We are uncertain whether ivermectin compared to placebo reduces or increases viral clearance at seven days (RR 3.00, 95% CI 0.13 to 67.06; 1 study, 24 participants; low-certainty evidence). Ivermectin may have little or no effect compared to placebo or standard of care on the number of participants with symptoms resolved up to 14 days (RR 1.04, 95% CI 0.89 to 1.21; 1 study, 398 participants; low-certainty evidence) and adverse events within 28 days (RR 0.95, 95% CI 0.86 to 1.05; 2 studies, 422 participants; low-certainty evidence). None of the studies reporting duration of symptoms were eligible for primary analysis. No study reported hospital admission or quality of life up to 14 days. Ivermectin compared to no treatment for prevention of SARS-CoV-2 infection We found one study. Mortality up to 28 days was the only outcome eligible for primary analysis. We are uncertain whether ivermectin reduces or increases mortality compared to no treatment (0 participants died; 1 study, 304 participants; very low-certainty evidence). The study reported results for development of COVID-19 symptoms and adverse events up to 14 days that were included in a secondary analysis due to high risk of bias. No study reported SARS-CoV-2 infection, hospital admission, and quality of life up to 14 days.
Authors' conclusions: Based on the current very low- to low-certainty evidence, we are uncertain about the efficacy and safety of ivermectin used to treat or prevent COVID-19. The completed studies are small and few are considered high quality. Several studies are underway that may produce clearer answers in review updates. Overall, the reliable evidence available does not support the use ivermectin for treatment or prevention of COVID-19 outside of well-designed randomized trials.
Copyright © 2021 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
MP: funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the CEOsys project, which was paid to the institution).
MS: none.
MIM: none.
SG: none.
PK: none.
PM: none.
NS: none.
SW: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the CEOsys project, which was paid to the institution).
Figures




























Comment in
-
Stamp out fake clinical data by working together.Nature. 2022 Jan;601(7892):167. doi: 10.1038/d41586-022-00025-6. Nature. 2022. PMID: 35017708 No abstract available.
References
References to studies included in this review
Ahmed 2020 {published data only}
Chaccour 2021 {published data only}
-
- Chaccour C, Casellas A, Blanco-Di Matteo A, Pineda I, Fernandez-Montero A, Ruiz-Castillo P, et al. The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with mild COVID-19: a pilot, double-blind, placebo-controlled, randomized clinical trial. researchsquare.com/article/rs-116547/v1 (first received 7 December 2020). [DOI: 10.21203/rs.3.rs-116547/v1] - DOI - PMC - PubMed
-
- Chaccour C, Casellas A, Blanco-Di Matteo A, Pineda I, Fernandez-Montero A, Ruiz-Castillo P, et al. The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: a pilot, double-blind, placebo-controlled, randomized clinical trial. EClinicalMedicine 2021;32:100720. [PMID: ] - PMC - PubMed
-
- Chaccour C, Ruiz-Castillo P, Richardson MA, Moncunill G, Casellas A, Carmona-Torre F, et al. The SARS-CoV-2 ivermectin Navarra-ISGlobal Trial (SAINT) to evaluate the potential of ivermectin to reduce COVID-19 transmission in low risk, non-severe COVID-19 patients in the first 48 hours after symptoms onset: a structured summary of a study protocol for a randomized control pilot trial. Trials 2020;21(1):498. [PMID: ] - PMC - PubMed
-
- EUCTR2020-001474-29-ES. Pilot study to evaluate the potential of ivermectin to reduce COVID-19 transmission. www.clinicaltrialsregister.eu/ctr-search/trial/2020-001474-29/ES (first received 8 May 2020).
-
- NCT04390022. Sars-CoV-2/COVID-19 ivermectin Navarra-ISGlobal Trial (SAINT). clinicaltrials.gov/ct2/show/NCT04390022 (first received 17 December 2020).
Chachar 2020 {published data only}
-
- Chachar AZ, Khan KA, Asif M, Tanveer K, Khaqan A, Basri R. Effectiveness of ivermectin in SARS-CoV-2/COVID-19 patients. International Journal of Sciences 2020;9:31-5. [DOI: 10.18483/ijSci.2378] - DOI
-
- NCT04739410. Effectiveness of ivermectin in SARS-CoV-2/COVID-19 patients. clinicaltrials.gov/ct2/show/NCT04739410 (first received 4 February 2021).
Gonzalez 2021 {published data only}
-
- Gonzalez BJ, González Gámez M, Enciso EA, Maldonado RJ, Palacios HP, Dueñas Campos S, et al. Efficacy and safety of ivermectin and hydroxychloroquine in patients with severe COVID-19. A randomized controlled trial. medrxiv.org/content/early/2021/02/23/2021.02.18.21252037 (first received 23 February 2021). [DOI: 10.1101/2021.02.18.21252037] - DOI - PubMed
-
- NCT04391127. Hydroxychloroquine and ivermectin for the treatment of COVID-19 infection. clinicaltrials.gov/ct2/show/NCT04391127 (first received 18 May 2020).
Kirti 2021 {published data only}
-
- CTRI/2020/08/027225. Ivermectin as a possible treatment for COVID-19. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=46660&EncHid=&am... (first received 18 August 2020).
-
- Kirti R, Roy R, Pattadar C, Raj R, Agarwal N, Biswas B, et al. Ivermectin as a potential treatment for mild to moderate COVID-19 – a double blind randomized placebo-controlled trial. medrxiv.org/content/10.1101/2021.01.05.21249310v1 (first received 9 January 2021). [DOI: 10.1101/2021.01.05.21249310] - DOI - PubMed
Kishoria 2020 {published data only}
-
- Kishoria N, Mathur SL, Parmar V, Kaur RJ, Agarwal H, Verma S. Ivermectin as adjuvant to hydroxychlorquine in patients resistant to standard treatment for SARS-CoV-2: results of an open-label randomized clinical study. Paripex — Indian Journal of Research 2020;9(8):4801859. [DOI: 10.36106/paripex/4801859] - DOI
Krolewiecki 2020 {published data only}
-
- Krolewiecki A, Lifschitz A, Moragas M, Travacio M, Valentini R, Alonso D, et al. Antiviral effect of high-dose ivermectin in adults with COVID-19: a pilot randomised, controlled, open label, multicentre trial. ssrn.com/abstract=3714649 (first received 11 November 2020). [DOI: 10.2139/ssrn.3714649] - DOI
-
- NCT04381884. Ivermectin effect on SARS-CoV-2 replication in patients with COVID-19. clinicaltrials.gov/ct2/show/NCT04381884 (first received 11 May 2020).
López‐Medina 2021 {published data only}
-
- NCT04405843. Efficacy of ivermectin in adult patients with early stages of COVID-19 (EPIC Trial) (EPIC). clinicaltrials.gov/ct2/show/NCT04405843 (first received 28 May 2020).
Mohan 2021 {published data only}
-
- CTRI/2020/06/026001. Ivermectin in COVID. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=44196&EncHid=&am... (first received 21 June 2020).
-
- Mohan A, Tiwari P, Suri T, Mittal S, Patel A, Jain A, et al. Ivermectin in mild and moderate COVID-19 (RIVET-COV): a randomized, placebo-controlled trial. researchsquare.com/article/rs-191648/v1 (first received 2 February 2021). [DOI: 10.21203/rs.3.rs-191648/v1] - DOI - PMC - PubMed
Okumuş 2021 {published data only}
-
- NCT04646109. Ivermectin for severe COVID-19 management. clinicaltrials.gov/ct2/show/NCT04646109 (first received 27 November 2020).
-
- Okumuş N, Demirtürk N, Çetinkaya RA, Güner R, Avcı IY, Orhan S, et al. Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients. researchsquare.com/article/rs-224203/v1 (first received 24 February 2021). [DOI: 10.21203/rs.3.rs-224203/v1] - DOI - PMC - PubMed
Podder 2020 {published data only}
-
- Podder CS, Chowdhury N, Sina MI, Haque WM. Outcome of ivermectin treated mild to moderate COVID-19 cases: a single-centre, open-label, randomised controlled study. IMC Journal of Medical Science 2020;14(2):11-8. [DOI: ]
Pott‐Junior 2021 {published data only}
-
- NCT04431466. A study to compare the efficacy and safety of different doses of ivermectin for COVID-19. clinicaltrials.gov/ct2/show/NCT04431466 (first received 16 June 2020).
Shah Bukhari 2021 {published data only}
-
- NCT04392713. Efficacy of ivermectin in COVID-19. clinicaltrials.gov/ct2/show/NCT04392713 (first received 19 May 2020).
-
- Shah Bukhari KH, Asghar A, Perveen N, Hayat A, Mangat SA, Butt KR, et al. Efficacy of ivermectin in COVID-19 patients with mild to moderate disease. medrxiv.org/content/early/2021/02/05/2021.02.02.21250840 (first received 5 February 2021). [DOI: 10.1101/2021.02.02.21250840] - DOI
Shoumann 2021 {published data only}
-
- NCT04422561. Prophylactic ivermectin in COVID-19 contacts. clinicaltrials.gov/ct2/show/NCT04422561 (first received 9 June 2020).
-
- Shoumann WM, Hegazy AA, Nafae RM, Ragab MI, Samra SR, Ibrahim DA, et al. Use of ivermectin as a potential chemoprophylaxis for COVID-19 in Egypt: a randomized clinical trial. Journal of Clinical and Diagnostic Research 2021;15(2):27-32. [DOI: 10.7860/JCDR/2021/46795.14529] - DOI
References to studies excluded from this review
Babalola 2021 {published data only}
-
- Babalola OE, Bode CO, Ajayi AA, Alakaloko FM, Akase IE, Otrofanowei E, et al. Ivermectin shows clinical benefits in mild to moderate COVID19: a randomised controlled double-blind, dose-response study in Lagos. Quarterly Journal of Medicine 2021 Feb 18 [Epub ahead of print]. [DOI: 10.1093/qjmed/hcab035] - DOI - PMC - PubMed
-
- Babalola OE, Bode CO, Ajayi AA, Alakaloko FM, Akase IE, Otrofanowei E, et al. Ivermectin shows clinical benefits in mild to moderate COVID19: a randomised controlled double blind dose response study in Lagos. medrxiv.org/content/10.1101/2021.01.05.21249131v1 (first received 6 January 2021). [DOI: 10.1101/2021.01.05.21249131] - DOI - PMC - PubMed
-
- ISRCTN40302986. Does ivermectin cure and/or prevent COVID-19? isrctn.com/ISRCTN40302986 (first received 23 April 2020). [DOI: 10.1186/ISRCTN40302986] - DOI
Behera 2020 {published data only}
-
- Behera P, Patro BK, Singh AK, Chandanshive PD, Ravi K, Pradhan SK, et al. Role of ivermectin in the prevention of COVID-19 infection among healthcare workers in India: a matched case-control study. 10.1101/2020.10.29.20222661 (first received 3 November 2020). [DOI: 10.1101/2020.10.29.20222661] - DOI
Cadegiani 2020 {published data only}
-
- Cadegiani F, Goren A, Wambier CG, McCoy J. Early COVID-19 therapy with azithromycin plus nitazoxanide, ivermectin or hydroxychloroquine in outpatient settings significantly reduced symptoms compared to known outcomes in untreated patients. medrxiv.org/content/10.1101/2020.10.31.20223883v1 (first received 4 November 2020). [DOI: 10.1101/2020.10.31.20223883] - DOI - PMC - PubMed
-
- Cadegiani F, Wambier CG, Goren A, McCoy J. Early COVID-19 therapy with azithromycin plus nitazoxanide, ivermectin or hydroxychloroquine in outpatient settings significantly reduced symptoms compared to known outcomes in untreated patients. researchsquare.com/article/rs-100994/v1 (first received 3 November 2020). [DOI: 10.21203/rs.3.rs-100994/v1] - DOI - PubMed
-
- Cadegiani F, Goren A, McCoy J, Wambier CG. Hydroxychloroquine, nitazoxanide and ivermectin have similar effects in early COVID-19: a head-to-head comparison of the Pre-AndroCoV Trial. researchsquare.com/article/rs-98106/v1 (first received 29 October 2020). [DOI: 10.21203/rs.3.rs-98106/v1] - DOI
-
- Cadegiani F, Goren A, Wambier CG, McCoy J. An open-label prospective observational study of antiandrogen and non-antiandrogen early pharmacological approaches in females with mild-to-moderate COVID-19. The Pre-AndroCoV Female Trial. medrxiv.org/content/10.1101/2020.10.05.20206870v1 (first received 6 October 2020). [DOI: 10.1101/2020.10.05.20206870] - DOI
Camprubi 2020 {published data only}
Carvallo 2020 {published data only}
-
- Carvallo H, Hirsch R, Farinella EM. Safety and efficacy of the combined use of ivermectin, dexamethasone, enoxaparin and aspirin against COVID 19. medrxiv.org/content/10.1101/2020.09.10.20191619v1 (first received 15 September 2020). [DOI: 10.1101/2020.09.10.20191619] - DOI
-
- NCT04425863. Ivermectin, aspirin, dexamethasone and enoxaparin as treatment of Covid 19 (IDEA). clinicaltrials.gov/ct2/show/NCT04425863 (first received 11 June 2020).
Chahla 2021a {published data only}
-
- Chahla RE, Medina Ruiz L, Mena T, Brepe Y, Terranova P, Ortega ES, et al. A randomized trial – intensive treatment based in ivermectin and iota-carrageenan as pre-exposure prophylaxis for COVID-19 in healthcare agents. medrxiv.org/content/10.1101/2021.03.26.21254398v1 (first received 30 March 2021). [DOI: 10.1101/2021.03.26.21254398v1] - DOI
-
- NCT04701710. Prophylaxis Covid-19 in healthcare agents by intensive treatment with ivermectin and Iota-carrageenan (Ivercar-Tuc). clinicaltrials.gov/ct2/show/NCT04701710 (first received 8 January 2021).
Chahla 2021b {published data only}
-
- Chahla RE, Medina Ruiz L, Mena T, Brepe Y, Terranova P, Ortega ES, et al. Cluster randomised trials – ivermectin repurposing for COVID-19 treatment of outpatients with mild disease In primary health care centers. researchsquare.com/article/rs-495945/v1 (first received 6 May 2021). [DOI: 10.21203/rs.3.rs-495945/v1] - DOI
-
- Chahla RE, Medina Ruiz L, Mena T, Brepe Y, Terranova P, Ortega ES, et al. Ivermectin reproposing for COVID-19 treatment outpatients in mild stage in primary health care centers. medrxiv.org/content/10.1101/2021.03.29.21254554v1 (first received 20 March 2021). [DOI: 10.1101/2021.03.29.21254554v1] - DOI
Chowdhury 2021 {published data only}
-
- Chowdhury AT, Shahbaz M, Karim R, Islam J, Dan G, Shuixiang H. A comparative study on ivermectin-doxycycline and hydroxychloroquine-azithromycin therapy on COVID-19 patients. Eurasian Journal of Medicine and Oncology 2021;5(1):63-70. [DOI: 10.14744/ejmo.2021.16263] - DOI
-
- Chowdhury AT, Shahbaz M, Karim R, Islam J, Dan G, Shuixiang H. A randomized trial of ivermectin-doxycycline and hydroxychloroquine-azithromycin therapy on COVID19 patients. researchsquare.com/article/rs-38896/v1 (first received 14 July 2020). [DOI: 10.21203/rs.3.rs-38896/v1] - DOI
-
- NCT04434144. A comparative study on ivermectin and hydroxychloroquine on the COVID19 patients in Bangladesh. clinicaltrials.gov/ct2/show/NCT04434144 (first received 16 June 2020).
CTRI/2020/08/027282 {published data only}
-
- CTRI/2020/08/027282. Prophylactic ivermectin in COVID 19 contacts. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=46676&EncHid=&am... (first received 20 August 2020).
CTRI/2020/08/027394 {published data only}
-
- CTRI/2020/08/027394. Assessment of response of ivermectin on virological clearance in COVID-19 patients. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=46873&EncHid=&am... (first received 26 August 2020).
CTRI/2020/10/028335 {published data only}
-
- CTRI/2020/10/028335. A clinical study to assess the efficacy and safety of Tinefcon in patients with moderate COVID-19 infection. cochranelibrary.com/es/central/doi/10.1002/central/CN-02186249/full (first received 30 November 2020).
Elgazzar 2020 {published data only}
-
- Elgazzar A, Eltaweel A, Youssef SA, Hany B, Hafez M, Moussa H. Efficacy and safety of ivermectin for treatment and prophylaxis of COVID-19 pandemic (preprint). researchsquare.com/article/rs-100956/v3 (first received 28 December 2020). [DOI: 10.21203/rs.3.rs-100956/v3] - DOI
-
- NCT04668469. Efficacy and safety of ivermectin for treatment and prophylaxis of COVID-19 pandemic. clinicaltrials.gov/ct2/show/NCT04668469 (first received 16 December 2020).
Galan 2021 {published data only}
-
- Galan LE, Melo dos Santos N, Asato MS, Araújo JV, Moreira A, Marques-Araújo AM. Phase 2 randomized study on chloroquine, hydroxychloroquine or ivermectin in hospitalized patients with severe manifestations of SARS-CoV-2 infection. Pathology Global Health 2021;115(4):235-42. [DOI: 10.1080/20477724.2021.1890887] - DOI - PMC - PubMed
-
- RBR-8h7q82. The effect of chloroquine, hydroxychloroquine or ivermectin in patients with severe manifestations of coronavirus. ensaiosclinicos.gov.br/rg/RBR-8h7q82/ (first received 2 October 2020).
Gorial 2020 {published data only}
-
- Gorial FI, Mashhadani S, Sayaly HM, Dakhil BD, AlMashhadani MM, Aljabory AM, et al. Effectiveness of ivermectin as add-on therapy in COVID-19 management (pilot trial). medrxiv.org/content/10.1101/2020.07.07.20145979v1 (first received 8 July 2020). [DOI: 10.1101/2020.07.07.20145979] - DOI
-
- NCT04343092. Ivermectin adjuvant to hydroxychloroquin in COVID19 patients. clinicaltrials.gov/ct2/show/NCT04343092 (first received 4 November 2020).
Hashim 2020 {published data only}
-
- Hashim HA, Maulood MF, Rasheed AM, Fatak DF, Kabah KK, Abdulamir AS. Controlled randomized clinical trial on using Ivermectin with doxycycline for treating COVID-19 patients in Baghdad, Iraq. medrxiv.org/content/10.1101/2020.10.26.20219345v1 (first received 27 October 2020). [DOI: 10.1101/2020.10.26.20219345] - DOI
-
- NCT04591600. Effectiveness of ivermectin and doxycycline on COVID-19 patients. clinicaltrials.gov/ct2/show/NCT04591600 (first received 19 October 2020).
IRCT20180922041089N4 {published data only}
-
- IRCT20180922041089N4. Evaluation of the effect of oral Ivermectin on the outcome of patients with COVID-19 and compare it with the effect of conjunctional therapies in patients admitted to Ziaeian, Baharloo, Imam Khomeini in the spring and summer 2020. en.irct.ir/trial/50305 (first received 23 August 2020).
IRCT20200408046987N2 {published data only}
-
- IRCT20200408046987N2. Determination the therapeutic effect of Ivermectin and Sovodak on patients infected with COVID-19: a clinical trial. en.irct.ir/trial/51007 (first received 7 November 2020).
Lima‐Morales 2021 {published data only}
-
- Lima-Morales R, Méndez-Hernández P, Flores YN, Osorno-Romero P, Sancho-Hernándezk CR, Cuecuecha-Rugerio E. Effectiveness of a multidrug therapy consisting of ivermectin, azithromycin, montelukast, and acetylsalicylic acid to prevent hospitalization and death among ambulatory COVID-19 cases in Tlaxcala, Mexico. International Journal of Infectious Diseases 2021;105:598-605. [DOI: 10.1016/j.ijid.2021.02.014] - DOI - PMC - PubMed
Mahmud 2021 {published data only}
-
- NCT04523831. Clinical trial of ivermectin plus doxycycline for the treatment of confirmed Covid-19 infection. clinicaltrials.gov/ct2/show/results/NCT04523831 (first received 24 August 2020).
Morgenstern 2020 {published data only}
-
- Morgenstern J, Redondo JN, De León A, Canela JM, Torres N, Tavares J, et al. The use of compassionate Ivermectin in the management of symptomatic outpatients and hospitalized patients with clinical diagnosis of COVID-19 at the Medical Center Bournigal and the Medical Center Punta Cana, Rescue Group, Dominican Republic, from May 1 to August 10, 2020. medrxiv.org/content/10.1101/2020.10.29.20222505v1 (first received 3 November 2020). [DOI: 10.1101/2020.10.29.20222505] - DOI
NCT04345419 {published data only}
-
- NCT04345419. Remdesivir efficacy in coronavirus disease. clinicaltrials.gov/ct2/show/NCT04345419 (first received 14 April 2020).
NCT04360356 {published data only}
-
- NCT04360356. Ivermectin and nitazoxanide combination therapy for COVID-19. clinicaltrials.gov/ct2/show/NCT04360356 (first received 24 April 2020).
NCT04374279 {published data only}
-
- NCT04374279. Trial to promote recovery from COVID-19 with ivermectin or endocrine therapy. clinicaltrials.gov/ct2/show/NCT04374279 (first received 5 May 2020).
NCT04382846 {published data only}
-
- NCT04382846. Novel regimens in COVID-19 treatment. clinicaltrials.gov/ct2/show/NCT04382846 (first received 11 May 2020).
NCT04392427 {published data only}
-
- NCT04392427. New antiviral drugs for treatment of COVID-19. clinicaltrials.gov/ct2/show/NCT04392427 (first received 18 May 2020).
NCT04435587 {published data only}
-
- NCT04435587. Ivermectin vs combined hydroxychloroquine and antiretroviral drugs (ART) among asymptomatic COVID-19 infection (IDRA-COVID19). clinicaltrials.gov/ct2/show/NCT04435587 (first received 17 June 2020).
NCT04447235 {published data only}
-
- NCT04447235. Early treatment with ivermectin and losartan for cancer patients with COVID-19 Infection. clinicaltrials.gov/ct2/show/NCT04447235 (first received 25 June 2020).
NCT04482686 {published data only}
-
- NCT04482686. Trial of combination therapy to treat COVID-19 infection. clinicaltrials.gov/ct2/show/NCT04482686 (first received 22 July 2020).
NCT04530474 {published data only}
-
- NCT04530474. Outpatient use of ivermectin in COVID-19. clinicaltrials.gov/ct2/show/NCT04530474 (first received 28 August 2020).
NCT04551755 {published data only}
-
- NCT04551755. Safety and efficacy of ivermectin and doxycycline in treatment of Covid-19. clinicaltrials.gov/ct2/show/NCT04551755 (first received 16 September 2020).
NCT04703608 {published data only}
-
- NCT04703608. Prevention and treatment for COVID -19 (severe acute respiratory syndrome coronavirus 2 SARS-CoV-2) associated severe pneumonia in the Gambia (PaTS-COVID). clinicaltrials.gov/ct2/show/NCT04703608 (first received 11 January 2021).
NCT04723459 {published data only}
-
- NCT04723459. Efficacy of nano-ivermectin impregnated masks in prevention of Covid-19 among healthy contacts and medical staff. clinicaltrials.gov/ct2/show/NCT04723459 (first received 25 January 2021).
NCT04768179 {published data only}
-
- NCT04768179. Safety & efficacy of low dose aspirin/ivermectin combination therapy for treatment of Covid-19 patients (IVCOM). clinicaltrials.gov/ct2/show/NCT04768179 (first received 24 February 2021).
Niaee 2020 {published data only}
-
- IRCT20200408046987N1. Dose-finding study of Ivermectin treatment on patients infected with Covid-19: a clinical trial. en.irct.ir/trial/47012 (first received 27 April 2020).
-
- Niaee MS, Gheibi N, Namdar P, Allami A, Zolghadr L, Javadi A, et al. Ivermectin as an adjunct treatment for hospitalized adult COVID-19 patients: a randomized multi-center clinical trial (preprint). researchsquare.com/article/rs-109670/v1 (first received 24 November 2020). [DOI: 10.21203/rs.3.rs-109670/v1] - DOI
Rajter 2021 {published data only}
-
- Rajter JC, Sherman MS, Fatteh N, Vogel F, Sacks J, Rajter JJ. ICON (Ivermectin in COvid Nineteen) study: use of ivermectin is associated with lower mortality in hospitalized patients with COVID19. medrxiv.org/content/10.1101/2020.06.06.20124461v2 (first received 10 June 2020). [DOI: 10.1101/2020.06.06.20124461] - DOI - PubMed
Seet 2021 {published data only}
-
- NCT04446104. A preventive treatment for migrant workers at high-risk of COVID-19. clinicaltrials.gov/ct2/show/NCT04446104 (first received 24 June 2020).
-
- Seet RC, Lin Quek AM, Qin Ooi DS, Sengupta S, Lashminarasappa SR, Yang Koo C. Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: an open-label randomized trial. International Journal of Infectious Diseases 2021;106:314-22. [DOI: 10.1016/j.ijid.2021.04.035] - DOI - PMC - PubMed
Shahbaznejad 2021 {published data only}
-
- IRCT20111224008507N3. Effectiveness of ivermectin in the treatment of coronavirus infection in patients admitted to educational hospitals of Mazandaran in 2020. en.irct.ir/trial/49174 (first received 27 June 2020).
-
- Shahbaznejad L, Davoudi A, Eslami G, Markowitz JS, Navaeifar MR, Hosseinzadeh F, et al. Effects of ivermectin in patients with COVID-19: a multicenter, double-blind, randomized, controlled clinical trial. Clinical Therapeutics 6 May 2021 [Epub ahead of print]. [DOI: 10.1016/j.clinthera.2021.04.007] - DOI - PMC - PubMed
Spoorthi 2020 {published data only}
-
- Spoorthi V, Sasank S. Utility of ivermectin and doxycycline combination for the treatment of SARS-CoV-2. International Archives of Integrated Medicine 2020;7(10):177-82. [iaimjournal.com/wp-content/uploads/2020/10/iaim_2020_0710_23.pdf]
References to studies awaiting assessment
2020‐001971‐33/ES {published data only}
-
- 2020-001971-33/ES. Pragmatic study "CORIVER": ivermectin as antiviral treatment for patients infected by SARS-COV2 (COVID19). clinicaltrialsregister.eu/ctr-search/trial/2020-001971-33/ES (first received 22 July 2020).
2020‐002091‐12/BG {published data only}
-
- 2020-002091-12/BG. Multicenter, randomized, double-blind, placebo-controlled study investigating efficacy, safety and tolerability of ivermectin HUVE-19 in patients with proven SARS-CoV-2 infection (COVID-19) and manifested clinical symptoms. clinicaltrialsregister.eu/ctr-search/trial/2020-002091-12/BG (first received 5 May 2020).
CTRI/2020/04/024948 {published data only}
-
- CTRI/2020/04/024948. A clinical trial to study the effects of hydroxychloroquine, ciclesonide and ivermectin in treatment of moderate COVID-19 illness. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=43364 (first received 30 April 2020).
CTRI/2020/06/025960 {published data only}
-
- CTRI/2020/06/025960. To study effect of ivermectin drug in patients infected with SARS-CoV-2 virus. ctri.nic.in/Clinicaltrials/showallp.php?mid1=44373&EncHid=&userN... (first received 18 June 2020).
Faisal 2020 {published data only}
-
- Faisal R, Ali Shah SF, Hussain M. Potential use of azithromycin alone and in combination with ivermectin in fighting against the symptoms of COVID-19. Professional Medical Journal 2020;28(5):737-41. [DOI: 10.29309/TPMJ/2021.28.05.5867] - DOI
Hosseini 2021 {published data only}
-
- Hosseini FS, Malektojari A, Ghazizadeh S, Hassaniazad M, Davoodian P, Dadvand H, et al. The efficacy and safety of ivermectin in patients with mild and moderate COVID-19: a structured summary of a study protocol for a randomized controlled trial. Trials 2021;22(1):4. [DOI: 10.1186/s13063-020-04988-7] - DOI - PMC - PubMed
-
- IRCT20200506047323N6. The efficacy and safety of Ivermectin in patients with COVID-19: a randomized clinical trial. en.irct.ir/trial/49501 (first received 17 November 2020).
IRCT20190602043787N3 {published data only}
-
- IRCT20190602043787N3. Evaluation of the effect of ivermectin in hospitalized patients with COVID-19 in Imam Reza Hospital in Mashhad. en.irct.ir/trial/49180 (first received 20 July 2020).
IRCT20200408046987N3 {published data only}
-
- IRCT20200408046987N3. Evaluation of prophylaxis induced by ivermectin in populations exposed to COVID-19 patients. www.irct.ir/trial/51999 (first received 6 December 2020).
IRCT20200422047168N2 {published data only}
-
- IRCT20200422047168N2. Clinical trial study of the therapeutic effect of ivermectin, besides kaletra and chloroquine in patients with coronavirus disease 2019 (COVID-19). en.irct.ir/trial/48444 (first received 30 May 2020).
ISRCTN90437126 {published data only}
-
- ISRCTN90437126 . Study on the effects of using ivermectin to prevent COVID-19 in an adult population in Brazil. www.isrctn.com/ISRCTN90437126 (first received 11 November 2020).
NCT04351347 {published data only}
-
- NCT04351347. The efficacy of ivermectin in larger doses in COVID-19 treatment. clinicaltrials.gov/ct2/show/NCT04351347 (first received 17 April 2020).
NCT04374019 {published data only}
-
- NCT04374019. Novel agents for treatment of high-risk COVID-19 positive patients. clinicaltrials.gov/ct2/show/NCT04374019 (first received 5 May 2020).
NCT04407130 {published data only}
-
- NCT04407130. Efficacy and safety of ivermectin and doxycycline in combination or IVE alone in patients with COVID-19 infection. clinicaltrials.gov/ct2/show/NCT04407130 (first received 29 May 2020).
NCT04407507 {published data only}
-
- NCT04407507. Efficacy, safety and tolerability of ivermectin in subjects infected with SARS-CoV-2 with or without symptoms. clinicaltrials.gov/ct2/show/NCT04407507 (first received 29 May 2020).
NCT04716569 {published data only}
-
- NCT04716569. Evaluation of ivermectin mucoadhesive nanosuspension as nasal spray in management of early Covid-19. clinicaltrials.gov/ct2/show/NCT04716569 (first received 20 January 2020).
NCT04746365 {published data only}
-
- NCT04746365. Ivermectin Role In Covid-19 Clinical Trial (IRICT). clinicaltrials.gov/ct2/show/NCT04746365 (first received 9 February 2021).
NCT04891250 {published data only}
-
- NCT04891250. The Zambia Ivermectin Trial for the treatment and prevention of COVID-19 (ZIT). clinicaltrials.gov/ct2/show/NCT04891250 (first received 18 May 2021).
Samaha 2021 {published data only}
-
- ChiCTR2000033627. In vivo use of ivermectin (IVR) for treatment for corona virus infected patients: a randomized controlled trial. chictr.org.cn/showprojen.aspx?proj=54707 (first received 7 June 2020).
References to ongoing studies
2020‐001994‐66/ES {published data only}
-
- 2020-001994-66/ES. Study of the efficacy of ivermectin in the treatment and prevention of COVID-19. clinicaltrialsregister.eu/ctr-search/trial/2020-001994-66/ES (first received 7 May 2020).
ACTRN12620000982910 {published data only}
-
- ACTRN12620000982910. A randomized double-blind placebo-controlled trial of oral ivermectin for outpatient treatment of those at high risk for hospitalization due to COVID-19. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12620000982910 (first received 14 September 2020).
CTRI/2020/05/025068 {published data only}
-
- CTRI/2020/05/025068. A phase IIB open label randomized controlled trial to evaluate the efficacy and safety of ivermectin in reducing viral loads in patients with hematological disorders who are admitted with COVID 19 infection. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=43449 (first received 7 May 2020).
CTRI/2020/05/025224 {published data only}
-
- CTRI/2020/05/025224. Study to efficacy of ivermectin in patients of COVID-19. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=43728&EncHid=&am... (first received 18 May 2020).
Garcia 2021 {published data only}
-
- Garcia PJ, Hurtado HM, Ugarte-Gil C, Leon P, Malaga G, Chaccour C, et al. Randomized clinical trial to compare the efficacy of ivermectin versus placebo to negativize nasopharyngeal PCR in patients with early COVID-19 in Peru (SAINT-Peru): a structured summary of a study protocol for randomized controlled trial. Trials 2021;22(1):262. [DOI: 10.1186/s13063-021-05236-2] [PMID: ] - DOI - PMC - PubMed
-
- Garica PJ, Huratdo HM, Urgae-Gil C, Leon P, Malaga G, Chaccour C, et al. Randomized clinical trial to compare the efficacy of ivermectin versus placebo to negativize nasopharyngeal PCR in patients with early COVID-19 in Peru (SAINT-Peru): a structured summary of a study protocol for randomized controlled trial. researchsquare.com/article/rs-345747/v1 (first received 23 May 2021). [DOI: 10.21203/rs.3.rs-345747/v1] - DOI - PMC - PubMed
-
- NCT04635943. Randomized phase IIA clinical trial to evaluate the efficacy of ivermectin to obtain negative PCR results in patients with early phase COVID-19 (SAINT-PERU). clinicaltrials.gov/ct2/show/NCT04635943 (first received 19 November 2020).
-
- PER-034-20. Randomized phase IIa clinical trial to compare the efficacy of ivermectin versus placebo to obtain negative PCR results in patients with early phase Covid-19. www.ins.gob.pe/ensayosclinicos/rpec/recuperarECPBNuevoEN.asp?numec=034-20 (first received 17 July 2020).
IRCT20111224008507N4 {published data only}
-
- IRCT20111224008507N4. Double-blind placebo-controlled clinical trial of evaluating the effectiveness of ivermectin in treatment of outpatients with COVID-19 in 2021. irct.ir/trial/53949 (first received 31 January 2021).
IRCT20111224008507N5 {published data only}
-
- IRCT20111224008507N5. Double-blind placebo-controlled clinical trial of evaluating the effectiveness of ivermectin in treatment of patients admitted with COVID-19 in 2021. en.irct.ir/trial/54402 (first received 22 February 2021).
IRCT20190624043993N2 {published data only}
-
- IRCT20190624043993N2. Evaluation effects of the standard regimen along with ivermectin on treatment of corona virus type 2 pneumonia. irct.ir/trial/49280 (first received 12 July 2020).
IRCT20200404046937N4 {published data only}
-
- IRCT20200404046937N4. Evaluating the efficacy and safety of Ivermectin in the treatment of COVID-19 patients: a double-blind randomized controlled trial, phase II. en.irct.ir/trial/49935 (first received 6 August 2020).
NCT04403555 {published data only}
-
- NCT04403555. Ivermectin as a novel therapy in COVID-19 treatment. clinicaltrials.gov/ct2/show/NCT04403555 (first received 27 May 2020).
NCT04425707 {published data only}
-
- NCT04425707. Ivermectin in treatment of COVID 19 patients. clinicaltrials.gov/ct2/show/NCT04425707 (first received 11 June 2020).
NCT04429711 {published data only}
-
- NCT04429711. Ivermectin vs. placebo for the treatment of patients with mild to moderate COVID-19. clinicaltrials.gov/ct2/show/NCT04429711 (first received 12 June 2020).
NCT04438850 {published data only}
-
- 2020-002283-32/IT. Randomized, double-blind, multi centre phase II, proof of concept, dose finding clinical trial on ivermectin for the early treatment of COVID-19. clinicaltrialsregister.eu/ctr-search/trial/2020-002283-32/IT (first received 10 August 2020).
-
- NCT04438850. COVidIVERmectin: ivermectin for treatment of Covid-19 (COVER). clinicaltrials.gov/ct2/show/NCT04438850 (first received 19 June 2020).
NCT04445311 {published data only}
-
- NCT04445311. Ivermectin in treatment of COVID-19. clinicaltrials.gov/ct2/show/NCT04445311 (first received 24 June 2020).
NCT04472585 {published data only}
-
- NCT04472585. Efficacy of subcutaneous ivermectin with or without zinc in COVID-19 patients (SIZI-COVID-PK). clinicaltrials.gov/ct2/show/NCT04472585 (first received 15 July 2020).
NCT04510233 {published data only}
-
- NCT04510233. Ivermectin nasal spray for COVID19 patients. clinicaltrials.gov/ct2/show/NCT04510233 (first received 12 August 2020).
NCT04527211 {published data only}
-
- NCT04527211. Effectiveness and safety of ivermectin for the prevention of Covid-19 infection in Colombian health personnel (IveprofCovid19). clinicaltrials.gov/ct2/show/NCT04527211 (first received 26 August 2020).
NCT04602507 {published data only}
-
- NCT04602507. Ivermectin in adults with severe COVID-19. clinicaltrials.gov/ct2/show/NCT04602507 (first received 26 October 2020).
NCT04673214 {published data only}
-
- NCT04673214. Evaluation of prognostic modification in COVID-19 patients in early intervention treatment, a randomized clinical trial. clinicaltrials.gov/ct2/show/NCT04673214 (first received 17 December 2020).
NCT04703205 {published data only}
-
- jRCT2031200120. Double-blind study in Covid-19 patients with ivermectin. jrct.niph.go.jp/en-latest-detail/jRCT2031200120 (first received 16 September 2020).
-
- NCT04703205. Study in Covid-19 patients with ivermectin (CORVETTE-01). clinicaltrials.gov/ct2/show/NCT04703205 (first received 11 January 2021).
NCT04712279 {published data only}
-
- NCT04712279. The (HD)IVACOV Trial (The High-Dose IVermectin Against COVID-19 Trial). https://clinicaltrials.gov/ct2/show/NCT04712279 (first received 15 January 2021).
NCT04727424 {published data only}
-
- NCT04727424. Repurposed approved therapies for outpatient treatment of patients with early-onset COVID-19 and mild symptoms. clinicaltrials.gov/ct2/show/NCT04727424 (first received 27 January 2021).
NCT04729140 {published data only}
-
- NCT04729140. An outpatient clinical trial using ivermectin and doxycycline in COVID-19 positive patients at high risk to prevent COVID-19 related hospitalization. clinicaltrials.gov/ct2/show/NCT04729140 (first received 28 January 2021).
NCT04834115 {published data only}
-
- NCT04834115. Efficacy of ivermectin in outpatients with non-severe COVID-19. clinicaltrials.gov/ct2/show/NCT04834115 (first received 6 April 2021).
NCT04836299 {published data only}
-
- NCT04836299. Clinical trial to "study the efficacy and therapeutic safety of ivermectin (SAINTBO). clinicaltrials.gov/ct2/show/NCT04836299 (first received 8 April 2021).
NCT04885530 {published data only}
-
- NCT04885530. ACTIV-6: COVID-19 study of repurposed medications. clinicaltrials.gov/ct2/show/NCT04885530 (first received 13 May 2021).
NCT04886362 {published data only}
-
- NCT04886362. Ivermectina Colombia (IVERCOL). clinicaltrials.gov/ct2/show/NCT04886362 (first received 14 May 2021).
NCT04894721 {published data only}
-
- NCT04894721. Prophylaxis for COVID-19: ivermectin in close contacts of COVID-19 cases (IVERNEX-TUC). clinicaltrials.gov/ct2/show/NCT04894721 (first received 20 May 2021).
PACTR202102588777597 {published data only}
-
- PACTR202102588777597. Ivermectin Treatment Trial (ITT). pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/re... (first received 12 February 2021).
PACTR202102848675636 {published data only}
-
- PACTR202102848675636. Double blind, community-based, randomized controlled trial on the use of ivermectin as post exposure chemo-prophylaxis for COVID-19 among high risk individuals in Lagos (IVERPEPCOV) COVID-19. pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/re... (first received 11 February 2021).
Vallejos 2020 {published data only}
-
- NCT04529525. Ivermectin to prevent hospitalizations in COVID-19 (IVERCORCOVID19). clinicaltrials.gov/ct2/show/NCT04529525 (first received 27 August 2020).
-
- Vallejos J, Zoni R, Bangher M, Villamandos S, Bobadilla A, Plano F, et al. Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19): a structured summary of a study protocol for a randomized controlled trial. Trials 2020;21(1):965. [DOI: 10.1186/s13063-020-04813-1] - DOI - PMC - PubMed
Additional references
Ashour 2019
Balshem 2011
-
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines – 3: rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401-6. [PMID: ] - PubMed
Beigel 2020
BIRD 2021
-
- British Ivermectin Recommendation Development panel. The BIRD recommendation on the use of ivermectin for Covid-19. francesoir.fr/sites/francesoir/files/media-icons/bird-proceedings-02-03-... (accessed prior to 19 July 2021).
Bryant 2021
Caly 2020
Campbell 1983
Cochrane LSR
-
- Cochrane LSR. Guidance for the production and publication of Cochrane living systematic reviews: Cochrane Reviews in living mode. Available from community.cochrane.org/review-production/production-resources/living-sys... (accessed 22 March 2021).
CONSORT 2010 Statement
-
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Medicine 2010;8:18. - PubMed
COVID Guidelines India 2021
-
- Covid Management Guidelines India Group. COVID Management Guidelines India. indiacovidguidelines.org/ivermectin/ (accessed 15 May 2021).
COVID‐NMA Working Group
-
- COVID-NMA working group. The COVID-NMA initiative: a living mapping and living systematic review of Covid-19 trials. covid-nma.com (accessed prior to 1 July 2021).
Deeks 2020
-
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane. Available from training.cochrane.org/handbook/archive/v6.1.
Deng 2020
Dong 2020
Dourmishev 2005
EMA 2021
-
- European Medicines Agency. EMA advises against use of ivermectin for the prevention or treatment of COVID-19 outside randomised clinical trials. ema.europa.eu/en/news/ema-advises-against-use-ivermectin-prevention-trea... (accessed 15 May 2021).
FDA 2020
-
- US Food and Drug Administration. Product safety information: COVID-19 and ivermectin intended for animals. fda.gov/animal-veterinary/product-safety-information/faq-covid-19-and-iv... (accessed 19 February 2020).
Garegnani 2021
German AWMF Guideline 2021
-
- Kluge S, Janssens U, Welte T, Weber-Carstens S, Schälte G, Spinner CD, et al. S3-Guideline – recommendations on Inpatient Treatment of Patients With COVID-19 [S3-Leitlinie - Empfehlungen zur stationären Therapie von Patienten mit COVID-19]. awmf.org/uploads/tx_szleitlinien/113-001l_S3_Empfehlungen-zur-stationaer... (accessed 25 February 2021). - PubMed
Goetz 2016
González‐Canga 2008
-
- González-Canga A, Sahagún-Prieto AM, Diez-Liébana MJ, Fernández-Martínez N, Sierra-Vega M, García-Vieitez JJ. The pharmacokinetics and interactions of ivermectin in humans. Journal of the American Association of Pharmaceutical Scientists 2008;10:42-6. [DOI: 10.1208/s12248-007-9000-9] - DOI - PMC - PubMed
Herrmann 2020
Higgins 2020a
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Higgins 2020b
-
- Higgins JP, Eldridge S, Li T. Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Higgins 2020c
-
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Higgins 2020d
-
- Higgins JP, Li T, Deeks JJ (editors). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Higgins 2021
-
- Higgins JP, Lasserson T, Chandler J, Tovey D, Thomas J, Flemyng E, et al. Methodological Expectations of Cochrane Intervention Reviews. Cochrane: London, Version February 2021. Available from: www.community.cochrane.org/mecir-manual/ (accessed prior to 19 July 2021).
Hill 2021
-
- Hill A, Abdulamir A, Ahmed S, Ashgar A, Babalola OE, Basri E, et al. Meta-analysis of randomized trials of ivermectin to treat SARS-CoV-2 infection. researchsquare.com/article/rs-148845/v1 (first received 19 January 2021). [DOI: 10.21203/rs.3.rs-148845/v1] - DOI
Huang 2020
IDSA 2021
-
- Bhimraj A, Morgan RL, Shumaker AH, Lavergne V, Baden L, Cheng VC, et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19, version 4.3.0. idsociety.org/practice-guideline/covid-19-guideline-treatment-and-manage... (accessed 15 May 2021).
IntHout 2016
ivmmeta.com
-
- Ivermectin for COVID-19: real-time meta analysis of 60 studies. ivmmeta.com/ (accessed prior to 1 July 2021).
Karagiannidis 2020
-
- Karagiannidis C, Mostert C, Hentschker C, Voshaar T, Malzahn J, Schillinger G, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respiratory Medicine 2020;9:853-62. [DOI: 10.1016/S2213-2600(20)30316-7] [PMID: ] - DOI - PMC - PubMed
Kobayashi 2020
Kory 2021
-
- Kory P, Meduri GU, Iglesias J, Varon J, Berkowitz K, Kornfeld H, et al. Review of the emerging evidence demonstrating the efficacy of ivermectin in the prophylaxis and treatment of COVID-19. osf.io/wx3zn/ (first received 16 January 2021). [DOI: 10.31219/osf.io/wx3zn] - DOI - PMC - PubMed
Lefebvre 2020
-
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf MI, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from: training.cochrane.org/handbook/archive/v6.1.
MAGICapp [Computer program]
-
- MAGICapp. MAGIC, 2020. Available at magicevidence.org/magicapp/.
Marshall 2020
Merck 2021
-
- Merck. Merck statement on ivermectin use during the COVID-19 pandemic. merck.com/news/merck-statement-on-ivermectin-use-during-the-covid-19-pan... (accessed 15 May 2021).
Meta [Computer program]
-
- cran.r-project.org/web/packages/meta Meta: general package for meta-analysis. cran.r-project.org/web/packages/meta, 2021. Available at cran.r-project.org/web/packages/meta/meta.pdf.
NIH 2021
-
- COVID-19 treatment guidelines panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. www.covid19treatmentguidelines.nih.gov/ (accessed 19 February 2021).
Oran 2021
Panahi 2015
-
- Panahi Y, Poursaleh Z, Goldust M. The efficacy of topical and oral ivermectin in the treatment of human scabies. Annals of Parasitology 2015;61(1):11-6. [PMID: ] - PubMed
Prescott 2020
PRINCIPLE trial
-
- University of Oxford. Ivermectin to be investigated in adults aged 18+ as a possible treatment for COVID-19 in the PRINCIPLE trial. principletrial.org/news/ivermectin-to-be-investigated-as-a-possible-trea... (accessed 1 July 2021).
RECOVERY 2021
RevMan Web 2020 [Computer program]
-
- Review Manager Web (RevMan Web). Version 2.3.0. Cochrane, 2020. Available at revman.cochrane.org.
Rodríguez‐Mega 2020
Schünemann 2020
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Siemieniuk 2020
Singh 2021
Sterne 2019
Supplementary File_Ivermectin_Risk of Bias
-
- Weibel S, Popp M. Supplementary File_Ivermectin_Risk of Bias Excel Tool (Version 1). Zenodo 2021. [DOI: 10.5281/zenodo.5118956] - DOI
Tay 2013
-
- Tay MY, Fraser JE, Chan WK, Moreland NJ, Rathore AP, Wang C, et al. Nuclear localization of dengue virus (DENV) 1–4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin. Antiviral Research 2013;99(3):301-6. [DOI: 10.1016/j.antiviral.2013.06.002] - DOI - PubMed
The Guardian 2021
-
- Davey M. Huge study supporting ivermectin as Covid treatment withdrawn over ethical concerns. Available at www.theguardian.com/science/2021/jul/16/huge-study-supporting-ivermectin... (accessed 15 July 2021).
Wagstaff 2012
Watson 2020
WHO 2012
-
- World Health Organization. The World Health Organization Quality of Life (WHOQOL). www.who.int/publications/i/item/WHO-HIS-HSI-Rev.2012.03 (accessed 19 March 2021).
WHO 2019
-
- World Health Organization. WHO model list of essential medicines, 21st list. www.who.int/medicines/publications/essentialmedicines/en (accessed 19 February 2021).
WHO 2020a
-
- World Health Organization. Report of the WHO–China Joint Mission on Coronavirus Disease 2019 (COVID-19). www.who.int/publications-detail/report-of-the-who-china-joint-mission-on... (accessed 3 June 2021).
WHO 2020b
-
- World Health Organization. WHO coronavirus disease (COVID-19) dashboard. covid19.who.int/ (accessed 2 July 2021).
WHO 2020c
-
- World Health Organization. Antigen-detection in the diagnosis of SARS-CoV-2 infection using rapid immunoassays: interim guidance, 11 September 2020. Available at https://apps.who.int/iris/handle/10665/334253.
WHO 2021a
-
- World Health Organization. Weekly epidemiological update – 19 January 2021. www.who.int/publications/m/item/weekly-epidemiological-update---19-janua... (accessed 19 February 2021).
WHO 2021b
-
- World Health Organization. Therapeutics and COVID-19. Living guideline. who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2021.1 (accessed 3 June 2021).
Williamson 2020
Wulan 2015
Yamasmith 2018
-
- Yamasmith E, Saleh-arong FA, Avirutnan P, Angkasekwinai N, Mairiang D, Wongsawat E, et al. Efficacy and safety of ivermectin against dengue infection: a phase III, randomized, double-blind, placebo-controlled trial. Internal Medicine and One Health. 34th Annual Meeting of the Royal College of Physicians of Thailand; 2018 April 26-28; Chonburi (THA) 2018.
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous


