Pulmonary surveillance in pediatric hematopoietic stem cell transplant: A multinational multidisciplinary survey
- PMID: 34319008
- PMCID: PMC9124519
- DOI: 10.1002/cnr2.1501
Pulmonary surveillance in pediatric hematopoietic stem cell transplant: A multinational multidisciplinary survey
Abstract
Background: Hematopoietic Stem Cell Transplant (HSCT) is an established treatment for malignant and non-malignant conditions and pulmonary disease is a leading cause of late term morbidity and mortality. Accurate and early detection of pulmonary complications is a critical step in improving long term outcomes. Existing guidelines for surveillance of pulmonary complications post-HSCT contain conflicting recommendations.
Aim: To determine the breadth of current practice in monitoring for pulmonary complications of pediatric HSCT.
Methods: An institutional review board approved, online, anonymous multiple-choice survey was distributed to HSCT and pulmonary physicians from the United States of America and Australasia using the REDcap platform. The survey was developed by members of the American Thoracic Society Working Group on Complications of Childhood Cancer, and was designed to assess patient management and service design.
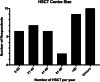
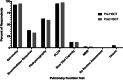
Results: A total of 40 (34.8%) responses were received. The majority (62.5%) were pulmonologists, and 82.5% were from the United States of America. In all, 67.5% reported having a protocol for monitoring pulmonary complications and 50.0% reported adhering "well" or "very well" to protocols. Pulmonary function tests (PFTs) most commonly involved spirometry and diffusion capacity for carbon monoxide. The frequency of PFTs varied depending on time post-HSCT and presence of complications. In all, 55.0% reported a set threshold for a clinically significant change in PFT.
Conclusions: These results illustrate current variation in surveillance for pulmonary complications of pediatric HSCT. The results of this survey will inform development of future guidelines for monitoring of pulmonary complications after pediatric HSCT.
Keywords: diagnostic screening programs; pediatrics; respiratory tract diseases; stem cell transplantation.
© 2021 The Authors. Cancer Reports published by Wiley Periodicals LLC.
Conflict of interest statement
The authors have no conflicts to disclose.
Figures
Similar articles
-
Trends and Clinical Implications of Pediatric Pulmonary Function After Hematopoietic Stem Cell Transplantation: A Systematic Review.Health Sci Rep. 2025 Jan 22;8(1):e70365. doi: 10.1002/hsr2.70365. eCollection 2025 Jan. Health Sci Rep. 2025. PMID: 39846049 Free PMC article. Review.
-
Detection of Bronchiolitis Obliterans Syndrome after Pediatric Hematopoietic Stem Cell Transplantation: An Official American Thoracic Society Clinical Practice Guideline.Am J Respir Crit Care Med. 2024 Aug 1;210(3):262-280. doi: 10.1164/rccm.202406-1117ST. Am J Respir Crit Care Med. 2024. PMID: 38889365
-
Pulmonary complications following hematopoietic stem cell transplantation in children.Turk J Pediatr. 2019;61(1):59-60. doi: 10.24953/turkjped.2019.01.010. Turk J Pediatr. 2019. PMID: 31559723
-
Lung function and late pulmonary complications among survivors of hematopoietic stem cell transplantation during childhood.Paediatr Respir Rev. 2010 Jun;11(2):115-22. doi: 10.1016/j.prrv.2010.01.006. Epub 2010 Apr 9. Paediatr Respir Rev. 2010. PMID: 20416548 Review.
-
Effectiveness of long-term routine pulmonary function surveillance following pediatric hematopoietic stem cell transplantation.Pediatr Pulmonol. 2014 Nov;49(11):1124-32. doi: 10.1002/ppul.22944. Epub 2013 Nov 4. Pediatr Pulmonol. 2014. PMID: 24574432
Cited by
-
Comparative longitudinal analysis of pulmonary function post-pediatric Allo-HSCT: benign vs. malignant diseases and early predictors.Ann Hematol. 2025 Aug 8. doi: 10.1007/s00277-025-06537-1. Online ahead of print. Ann Hematol. 2025. PMID: 40779049
-
Multiple breath washout and oscillometry after allogenic HSCT: a scoping review.Eur Respir Rev. 2023 Jul 26;32(169):220251. doi: 10.1183/16000617.0251-2022. Print 2023 Sep 30. Eur Respir Rev. 2023. PMID: 37495248 Free PMC article.
-
Pulmonary function testing in pediatric allogeneic stem cell transplant recipients to monitor for Bronchiolitis obliterans syndrome: a systematic review.BMC Pediatr. 2025 Mar 28;25(1):250. doi: 10.1186/s12887-025-05501-2. BMC Pediatr. 2025. PMID: 40155876 Free PMC article.
-
Trends and Clinical Implications of Pediatric Pulmonary Function After Hematopoietic Stem Cell Transplantation: A Systematic Review.Health Sci Rep. 2025 Jan 22;8(1):e70365. doi: 10.1002/hsr2.70365. eCollection 2025 Jan. Health Sci Rep. 2025. PMID: 39846049 Free PMC article. Review.
-
Pulmonary complications after hematopoietic stem cell transplantation in children: a functional and tomographic evaluation.J Bras Pneumol. 2022 Sep 26;48(5):e20220134. doi: 10.36416/1806-3756/e20220134. J Bras Pneumol. 2022. PMID: 36169559 Free PMC article. No abstract available.
References
-
- Shenoy S, Gaziev J, Angelucci E, et al. Late effects screening guidelines after hematopoietic cell transplantation (HCT) for hemoglobinopathy: consensus statement from the Second Pediatric Blood and Marrow Transplant Consortium International Conference on Late Effects after Pediatric HCT. Biol Blood Marrow Transplant. 2018;24(7):1313‐1321. 10.1016/j.bbmt.2018.04.002 - DOI - PubMed
-
- World Health Organization . Haematopoietic Stem Cell Transplantation. https://www.who.int/transplantation/hsctx/en/.
-
- D'Souza A, Fretham C. Current Uses and Outcomes of Hematopoietic Cell Transplantation (HCT): CIBMTR Summary Slides.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical