Nitric Oxide Photo-Donor Hybrids of Ciprofloxacin and Norfloxacin: A Shift in Activity from Antimicrobial to Anticancer Agents
- PMID: 34319100
- PMCID: PMC8389907
- DOI: 10.1021/acs.jmedchem.1c00917
Nitric Oxide Photo-Donor Hybrids of Ciprofloxacin and Norfloxacin: A Shift in Activity from Antimicrobial to Anticancer Agents
Erratum in
-
Correction to "Nitric Oxide Photo-Donor Hybrids of Ciprofloxacin and Norfloxacin: A Shift in Activity from Antimicrobial to Anticancer Agents".J Med Chem. 2022 Aug 25;65(16):11414. doi: 10.1021/acs.jmedchem.2c01209. Epub 2022 Aug 10. J Med Chem. 2022. PMID: 35947791 Free PMC article. No abstract available.
Abstract
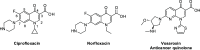
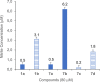
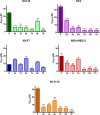
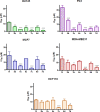
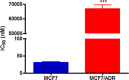
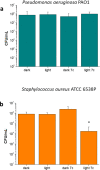
The potential anticancer effect of fluoroquinolone antibiotics has been recently unveiled and related to their ability to interfere with DNA topoisomerase II. We herein envisioned the design and synthesis of novel Ciprofloxacin and Norfloxacin nitric oxide (NO) photo-donor hybrids to explore the potential synergistic antitumor effect exerted by the fluoroquinolone scaffold and NO eventually produced upon light irradiation. Anticancer activity, evaluated on a panel of tumor cell lines, showed encouraging results with IC50 values in the low micromolar range. Some compounds displayed intense antiproliferative activity on triple-negative and doxorubicin-resistant breast cancer cell lines, paving the way for their potential use to treat aggressive, refractory and multidrug-resistant breast cancer. No significant additive effect was observed on PC3 and DU145 cells following NO release. Conversely, antimicrobial photodynamic experiments on both Gram-negative and Gram-positive microorganisms displayed a significant killing rate in Staphylococcus aureus, accounting for their potential effectiveness as selective antimicrobial photosensitizers.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
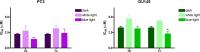

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

