High-Throughput Multiplex SARS-CoV-2 IgG Microsphere Immunoassay for Dried Blood Spots: A Public Health Strategy for Enhanced Serosurvey Capacity
- PMID: 34319133
- PMCID: PMC8552730
- DOI: 10.1128/Spectrum.00134-21
High-Throughput Multiplex SARS-CoV-2 IgG Microsphere Immunoassay for Dried Blood Spots: A Public Health Strategy for Enhanced Serosurvey Capacity
Abstract
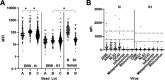
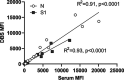
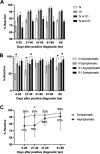
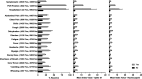
Early in the pandemic when diagnostic testing was not widely available, serosurveys played an important role in estimating the prevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in different populations. Dried blood spots (DBS), which can be collected in nonclinical settings, provide a minimally invasive alternative to serum for serosurveys. We developed a Luminex-based SARS-CoV-2 microsphere immunoassay (MIA) for DBS that detects IgG antibodies to nucleocapsid (N) and spike subunit 1 (S1) antigens. The assay uses a 384-well plate format and automated liquid handlers for high-throughput capacity. Specificity was assessed using a large collection of prepandemic DBS and well-characterized sera. Sensitivity was analyzed using serology data from New York State SARS-CoV-2 serosurvey testing and matched diagnostic test results. For DBS, the specificity was 99.5% for the individual N and S1 antigens. Median fluorescence intensity (MFI) values for DBS and paired sera showed a strong positive correlation for N (R2 = 0.91) and S1 (R2 = 0.93). Sensitivity, assessed from 1,134 DBS with prior laboratory-confirmed SARS-CoV-2 infection, ranged from 83% at 0 to 20 days to 95% at 61 to 90 days after a positive test. When stratified using coronavirus disease 2019 (COVID-19) symptom data, sensitivity ranged from 90 to 96% for symptomatic and 77 to 91% for asymptomatic individuals. For 8,367 health care workers reporting detailed symptom data, MFI values were significantly higher for all symptom categories. Our results indicate that the SARS-CoV-2 IgG DBS MIA is sensitive, specific, and well-suited for large population-based serosurveys. The ability to readily modify and multiplex antigens is important for ongoing assessment of SARS-CoV-2 antibody responses to emerging variants and vaccines. IMPORTANCE Testing for antibodies to SARS-CoV-2 has been used to estimate the prevalence of COVID-19 in different populations. Seroprevalence studies, or serosurveys, were especially useful during the early phase of the pandemic when diagnostic testing was not widely available, and the resulting seroprevalence estimates played an important role in public health decision making. To achieve meaningful results, antibody tests used for serosurveys should be accurate and accessible to diverse populations. We developed a test that detects antibodies to two different SARS-CoV-2 proteins in dried blood spots (DBS). DBS require only a simple fingerstick and can be collected in nonclinical settings. We conducted a robust validation study and have demonstrated that our test is both sensitive and specific. Furthermore, we demonstrated that our test is suitable for large-scale serosurveys by testing over 56,000 DBS collected in a variety of community-based venues in New York State during the spring of 2020.
Keywords: COVID-19; SARS-CoV-2; dried blood spot; immunoassays; serology; serosurvey.
Figures

Similar articles
-
Use of Self-Collected Dried Blood Spots and a Multiplex Microsphere Immunoassay to Measure IgG Antibody Response to COVID-19 Vaccines.Microbiol Spectr. 2023 Feb 14;11(1):e0133622. doi: 10.1128/spectrum.01336-22. Epub 2023 Jan 9. Microbiol Spectr. 2023. PMID: 36622204 Free PMC article.
-
Comparison of Dried Blood Spots and Venous Blood for the Detection of SARS-CoV-2 Antibodies in a Population of Nursing Home Residents.Microbiol Spectr. 2021 Oct 31;9(2):e0017821. doi: 10.1128/Spectrum.00178-21. Epub 2021 Sep 22. Microbiol Spectr. 2021. PMID: 34549995 Free PMC article.
-
Utility of Newborn Dried Blood Spots to Ascertain Seroprevalence of SARS-CoV-2 Antibodies Among Individuals Giving Birth in New York State, November 2019 to November 2021.JAMA Netw Open. 2022 Aug 1;5(8):e2227995. doi: 10.1001/jamanetworkopen.2022.27995. JAMA Netw Open. 2022. PMID: 35994287 Free PMC article.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2020 Jun 25;6(6):CD013652. doi: 10.1002/14651858.CD013652. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2022 Nov 17;11:CD013652. doi: 10.1002/14651858.CD013652.pub2. PMID: 32584464 Free PMC article. Updated.
-
SARS-CoV-2 antibody testing for estimating COVID-19 prevalence in the population.Cell Rep Med. 2021 Feb 16;2(2):100191. doi: 10.1016/j.xcrm.2021.100191. Epub 2021 Jan 14. Cell Rep Med. 2021. PMID: 33521694 Free PMC article. Review.
Cited by
-
Development of a novel serological assay for the detection of mpox infection in vaccinated populations.J Med Virol. 2023 Oct;95(10):e29134. doi: 10.1002/jmv.29134. J Med Virol. 2023. PMID: 37805977 Free PMC article.
-
Integrated Serosurveillance of Infectious Diseases Using Multiplex Bead Assays: A Systematic Review.Trop Med Infect Dis. 2025 Jan 10;10(1):19. doi: 10.3390/tropicalmed10010019. Trop Med Infect Dis. 2025. PMID: 39852670 Free PMC article. Review.
-
Longitudinal analysis of passively and actively acquired SARS-CoV-2 antibodies in infants with repeat newborn screening samples.Sci Rep. 2025 Jul 4;15(1):23881. doi: 10.1038/s41598-025-09140-6. Sci Rep. 2025. PMID: 40615534 Free PMC article.
-
Development of a Method for Detection of SARS-CoV-2 Nucleocapsid Antibodies on Dried Blood Spot by DELFIA Immunoassay.Diagnostics (Basel). 2023 Feb 27;13(5):897. doi: 10.3390/diagnostics13050897. Diagnostics (Basel). 2023. PMID: 36900041 Free PMC article.
-
Quantitating SARS-CoV-2 neutralizing antibodies from human dried blood spots.Microbiol Spectr. 2024 Oct 29;12(12):e0084624. doi: 10.1128/spectrum.00846-24. Online ahead of print. Microbiol Spectr. 2024. PMID: 39470282 Free PMC article.
References
-
- Gronvall G, Connell N, Farley JE, Inglesby T, Jennings JM, Mehta SH, West R, Kobokovich A. 2020. Developing a national strategy for SARS-CoV-2 serosurveys in the United States. Johns Hopkins Center for Health Security, Baltimore, MD. https://www.centerforhealthsecurity.org/our-work/publications/developing.... Accessed 25 April 2021.
-
- Centers for Disease Control and Prevention. 2017. Shipping guidelines for dried-blood spot specimens. https://www.cdc.gov/labstandards//pdf/nsqap/Bloodspot_Transportation_Gui.... Accessed April 25, 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

