Characterization of the Glutathione S-Transferases Involved in Styrene Degradation in Gordonia rubripertincta CWB2
- PMID: 34319142
- PMCID: PMC8552685
- DOI: 10.1128/Spectrum.00474-21
Characterization of the Glutathione S-Transferases Involved in Styrene Degradation in Gordonia rubripertincta CWB2
Abstract
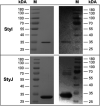
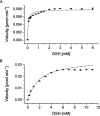
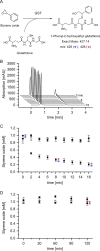
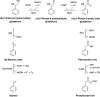
The glutathione S-transferases carried on the plasmid for the styrene-specific degradation pathway in the Actinobacterium Gordonia rubripertincta CWB2 were heterologously expressed in Escherichia coli. Both enzymes were purified via affinity chromatography and subjected to activity investigations. StyI and StyJ displayed activity toward the commonly used glutathione S-transferase model substrate 1-chloro-2,4-dinitrobenzene (CDNB) with Km values of 0.0682 ± 0.0074 and 2.0281 ± 0.1301 mM and Vmax values of 0.0158 ± 0.0002 and 0.348 ± 0.008 U mg-1 for StyI and StyJ, respectively. The conversion of the natural substrate styrene oxide to the intermediate (1-phenyl-2-hydroxyethyl)glutathione was detected for StyI with 48.3 ± 2.9 U mg-1. This elucidates one more step in the not yet fully resolved styrene-specific degradation pathway of Gordonia rubripertincta CWB2. A characterization of both purified enzymes adds more insight into the scarce research field of actinobacterial glutathione S-transferases. Moreover, a sequence and phylogenetic analysis puts both enzymes into a physiological and evolutionary context. IMPORTANCE Styrene is a toxic compound that is used at a large scale by industry for plastic production. Bacterial degradation of styrene is a possibility for bioremediation and pollution prevention. Intermediates of styrene derivatives degraded in the styrene-specific pathways are precursors for valuable chemical compounds. The pathway in Gordonia rubripertincta CWB2 has proven to accept a broader substrate range than other bacterial styrene degraders. The enzymes characterized in this study, distinguish CWB2s pathway from other known styrene degradation routes and thus might be the main key for its ability to produce ibuprofen from the respective styrene derivative. A biotechnological utilization of this cascade could lead to efficient and sustainable production of drugs, flavors, and fragrances. Moreover, research on glutathione metabolism in Actinobacteria is rare. Here, a characterization of two glutathione S-transferases of actinobacterial origin is presented, and the utilization of glutathione in the metabolism of an Actinobacterium is proven.
Keywords: Actinobacteria; biotransformation; glutathione; glutathione S-transferases; microbial ibuprofen production; styrene metabolism; styrene oxide.
Figures
References
-
- Tischler D. 2015. Microbial styrene degradation. Springer International Publishing, Basel, Switzerland. doi:10.1007/978-3-319-24862-2. - DOI
-
- Tischler D, Kaschabek S. 2012. Microbial styrene degradation: from basics to biotechnology, p 67–99. In Singh S (ed), Microbial degradation of xenobiotics. Springer, Berlin, Germany. doi:10.1007/978-3-642-23789-8_3. - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

