Mating Systems in True Morels (Morchella)
- PMID: 34319143
- PMCID: PMC8483713
- DOI: 10.1128/MMBR.00220-20
Mating Systems in True Morels (Morchella)
Abstract
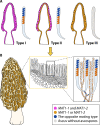
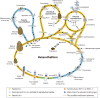
True morels (Morchella spp., Morchellaceae, Ascomycota) are widely regarded as a highly prized delicacy and are of great economic and scientific value. Recently, the rapid development of cultivation technology and expansion of areas for artificial morel cultivation have propelled morel research into a hot topic. Many studies have been conducted in various aspects of morel biology, but despite this, cultivation sites still frequently report failure to fruit or only low production of fruiting bodies. Key problems include the gap between cultivation practices and basic knowledge of morel biology. In this review, in an effort to highlight the mating systems, evolution, and life cycle of morels, we summarize the current state of knowledge of morel sexual reproduction, the structure and evolution of mating-type genes, the sexual process itself, and the influence of mating-type genes on the asexual stages and conidium production. Understanding of these processes is critical for improving technology for the cultivation of morels and for scaling up their commercial production. Morel species may well be good candidates as model species for improving sexual development research in ascomycetes in the future.
Keywords: asexual reproduction; evolution; genome analysis; heterothallism; mating type; mitospore; pseudohomothallism; skewed distribution; spatial competition; unisexual reproduction.
Figures



Similar articles
-
Artificial cultivation of true morels: current state, issues and perspectives.Crit Rev Biotechnol. 2018 Mar;38(2):259-271. doi: 10.1080/07388551.2017.1333082. Epub 2017 Jun 6. Crit Rev Biotechnol. 2018. PMID: 28585444 Review.
-
Phylogeny and historical biogeography of true morels (Morchella) reveals an early Cretaceous origin and high continental endemism and provincialism in the Holarctic.Fungal Genet Biol. 2011 Mar;48(3):252-65. doi: 10.1016/j.fgb.2010.09.006. Epub 2010 Oct 1. Fungal Genet Biol. 2011. PMID: 20888422
-
Opposite Polarity Monospore Genome De Novo Sequencing and Comparative Analysis Reveal the Possible Heterothallic Life Cycle of Morchella importuna.Int J Mol Sci. 2018 Aug 25;19(9):2525. doi: 10.3390/ijms19092525. Int J Mol Sci. 2018. PMID: 30149649 Free PMC article.
-
Large-scale commercial cultivation of morels: current state and perspectives.Appl Microbiol Biotechnol. 2022 Jun;106(12):4401-4412. doi: 10.1007/s00253-022-12012-y. Epub 2022 Jun 22. Appl Microbiol Biotechnol. 2022. PMID: 35731306 Review.
-
Structural variation and phylogenetic analysis of the mating-type locus in the genus Morchella.Mycologia. 2019 Jul-Aug;111(4):551-562. doi: 10.1080/00275514.2019.1628553. Epub 2019 Jun 28. Mycologia. 2019. PMID: 31251705
Cited by
-
Population distribution characteristics of mating type genes and genetic stability in Morchella sextelata.Arch Microbiol. 2024 Sep 23;206(10):412. doi: 10.1007/s00203-024-04141-x. Arch Microbiol. 2024. PMID: 39313680
-
Construction of nucleus-directed fluorescent reporter systems and its application to verification of heterokaryon formation in Morchella importuna.Front Microbiol. 2022 Nov 21;13:1051013. doi: 10.3389/fmicb.2022.1051013. eCollection 2022. Front Microbiol. 2022. PMID: 36478869 Free PMC article.
-
Organization and Unconventional Integration of the Mating-Type Loci in Morchella Species.J Fungi (Basel). 2022 Jul 19;8(7):746. doi: 10.3390/jof8070746. J Fungi (Basel). 2022. PMID: 35887501 Free PMC article.
-
Transcriptomic and Metabolomic Profiles Provide Insights into the Red-Stipe Symptom of Morel Fruiting Bodies.J Fungi (Basel). 2023 Mar 18;9(3):373. doi: 10.3390/jof9030373. J Fungi (Basel). 2023. PMID: 36983541 Free PMC article.
-
Dynamics of soil microbiome throughout the cultivation life cycle of morel (Morchella sextelata).Front Microbiol. 2023 Feb 22;14:979835. doi: 10.3389/fmicb.2023.979835. eCollection 2023. Front Microbiol. 2023. PMID: 36910237 Free PMC article.
References
-
- Matočec N, Kušan I, Mrvoš D, Raguzin E. 2014. The autumnal occurrence of the vernal genus Morchella (Ascomycota, Fungi). Nat Croat 23:163–177.
-
- Taşkın H, Doğan HH, Büyükalaca S. 2015. Morchella galilaea, an autumn species from Turkey. Mycotaxon 130:215–221. 10.5248/130.215. - DOI
-
- Pilz D, McLain R, Alexander S, Villarreal-Ruiz L, Berch S, Wurtz TL, Parks CG, McFarlane E, Baker B, Molina R, Smith JE. 2007. Ecology and management of morels harvested from the forests of western North America. General Technical Report PNW-GTR-710. US Department of Agriculture, Forest Service, Pacific Northwest Research Station, Portland, OR.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

