Rare Coding Variants Associated With Electrocardiographic Intervals Identify Monogenic Arrhythmia Susceptibility Genes: A Multi-Ancestry Analysis
- PMID: 34319147
- PMCID: PMC8373440
- DOI: 10.1161/CIRCGEN.120.003300
Rare Coding Variants Associated With Electrocardiographic Intervals Identify Monogenic Arrhythmia Susceptibility Genes: A Multi-Ancestry Analysis
Abstract
Background: Alterations in electrocardiographic (ECG) intervals are well-known markers for arrhythmia and sudden cardiac death (SCD) risk. While the genetics of arrhythmia syndromes have been studied, relations between electrocardiographic intervals and rare genetic variation at a population level are poorly understood.
Methods: Using a discovery sample of 29 000 individuals with whole-genome sequencing from Trans-Omics in Precision Medicine and replication in nearly 100 000 with whole-exome sequencing from the UK Biobank and MyCode, we examined associations between low-frequency and rare coding variants with 5 routinely measured electrocardiographic traits (RR, P-wave, PR, and QRS intervals and corrected QT interval).
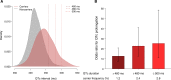
Results: We found that rare variants associated with population-based electrocardiographic intervals identify established monogenic SCD genes (KCNQ1, KCNH2, and SCN5A), a controversial monogenic SCD gene (KCNE1), and novel genes (PAM and MFGE8) involved in cardiac conduction. Loss-of-function and pathogenic SCN5A variants, carried by 0.1% of individuals, were associated with a nearly 6-fold increased odds of the first-degree atrioventricular block (P=8.4×10-5). Similar variants in KCNQ1 and KCNH2 (0.2% of individuals) were associated with a 23-fold increased odds of marked corrected QT interval prolongation (P=4×10-25), a marker of SCD risk. Incomplete penetrance of such deleterious variation was common as over 70% of carriers had normal electrocardiographic intervals.
Conclusions: Our findings indicate that large-scale high-depth sequence data and electrocardiographic analysis identifies monogenic arrhythmia susceptibility genes and rare variants with large effects. Known pathogenic variation in conventional arrhythmia and SCD genes exhibited incomplete penetrance and accounted for only a small fraction of marked electrocardiographic interval prolongation.
Keywords: death, sudden, cardiac; epidemiology; genetics; genome; population.
Figures


Similar articles
-
Monogenic and Polygenic Contributions to QTc Prolongation in the Population.Circulation. 2022 May 17;145(20):1524-1533. doi: 10.1161/CIRCULATIONAHA.121.057261. Epub 2022 Apr 7. Circulation. 2022. PMID: 35389749 Free PMC article.
-
Long QT syndrome type 5-Lite: Defining the clinical phenotype associated with the potentially proarrhythmic p.Asp85Asn-KCNE1 common genetic variant.Heart Rhythm. 2018 Aug;15(8):1223-1230. doi: 10.1016/j.hrthm.2018.03.038. Epub 2018 Apr 3. Heart Rhythm. 2018. PMID: 29625280 Free PMC article.
-
Association of Arrhythmia-Related Genetic Variants With Phenotypes Documented in Electronic Medical Records.JAMA. 2016 Jan 5;315(1):47-57. doi: 10.1001/jama.2015.17701. JAMA. 2016. PMID: 26746457 Free PMC article.
-
Genetics of sudden cardiac death.Curr Cardiol Rep. 2015 Jul;17(7):606. doi: 10.1007/s11886-015-0606-8. Curr Cardiol Rep. 2015. PMID: 26026997 Review.
-
Common genetic variants in sudden cardiac death.Heart Rhythm. 2009 Nov;6(11 Suppl):S3-9. doi: 10.1016/j.hrthm.2009.08.024. Epub 2009 Sep 1. Heart Rhythm. 2009. PMID: 19880071 Free PMC article. Review.
Cited by
-
A human FLII gene variant alters sarcomeric actin thin filament length and predisposes to cardiomyopathy.Proc Natl Acad Sci U S A. 2023 May 9;120(19):e2213696120. doi: 10.1073/pnas.2213696120. Epub 2023 May 1. Proc Natl Acad Sci U S A. 2023. PMID: 37126682 Free PMC article.
-
Monogenic and Polygenic Contributions to QTc Prolongation in the Population.Circulation. 2022 May 17;145(20):1524-1533. doi: 10.1161/CIRCULATIONAHA.121.057261. Epub 2022 Apr 7. Circulation. 2022. PMID: 35389749 Free PMC article.
-
Cardiac arrhythmias and genetics - current stage.Med Genet. 2025 Apr 8;37(2):125-136. doi: 10.1515/medgen-2025-2006. eCollection 2025 Jun. Med Genet. 2025. PMID: 40487301 Free PMC article.
-
High-throughput functional mapping of variants in an arrhythmia gene, KCNE1, reveals novel biology.Genome Med. 2024 May 30;16(1):73. doi: 10.1186/s13073-024-01340-5. Genome Med. 2024. PMID: 38816749 Free PMC article.
-
Meta-Analysis of Genome-Wide Association Studies Reveals Genetic Mechanisms of Supraventricular Arrhythmias.Circ Genom Precis Med. 2024 Jun;17(3):e004320. doi: 10.1161/CIRCGEN.123.004320. Epub 2024 May 28. Circ Genom Precis Med. 2024. PMID: 38804128 Free PMC article.
References
-
- Kurl S, Mäkikallio TH, Rautaharju P, Kiviniemi V, Laukkanen JA. Duration of QRS complex in resting electrocardiogram is a predictor of sudden cardiac death in men. Circulation. 2012;125:2588–2594. doi: 10.1161/CIRCULATIONAHA.111.025577 - PubMed
-
- Nielsen JB, Kühl JT, Pietersen A, Graff C, Lind B, Struijk JJ, Olesen MS, Sinner MF, Bachmann TN, Haunsø S, et al. . P-wave duration and the risk of atrial fibrillation: results from the Copenhagen ECG Study. Heart Rhythm. 2015;12:1887–1895. doi: 10.1016/j.hrthm.2015.04.026 - PubMed
Publication types
MeSH terms
Grants and funding
- R01 HL141901/HL/NHLBI NIH HHS/United States
- R01 HL046380/HL/NHLBI NIH HHS/United States
- K24 HL105780/HL/NHLBI NIH HHS/United States
- R35 HL135818/HL/NHLBI NIH HHS/United States
- MC_PC_17228/MRC_/Medical Research Council/United Kingdom
- R01 HL141989/HL/NHLBI NIH HHS/United States
- K01 HL135405/HL/NHLBI NIH HHS/United States
- CH/1996001/9454/BHF_/British Heart Foundation/United Kingdom
- R01 HL139731/HL/NHLBI NIH HHS/United States
- R01 HL092577/HL/NHLBI NIH HHS/United States
- MC_QA137853/MRC_/Medical Research Council/United Kingdom
- K24 HL148521/HL/NHLBI NIH HHS/United States
- 18SFRN34250007/AHA/American Heart Association-American Stroke Association/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

