A Novel Small Molecule Inhibits Hepatitis C Virus Propagation in Cell Culture
- PMID: 34319169
- PMCID: PMC8552720
- DOI: 10.1128/Spectrum.00439-21
A Novel Small Molecule Inhibits Hepatitis C Virus Propagation in Cell Culture
Abstract
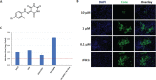
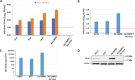
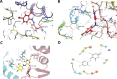
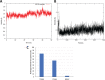
Hepatitis C virus (HCV) can cause acute and chronic infection that is associated with considerable liver-related morbidity and mortality. In recent years, there has been a shift in the treatment paradigm with the discovery and approval of agents that target specific proteins vital for viral replication. We employed a cell culture-adapted strain of HCV and human hepatoma-derived cells lines to test the effects of our novel small-molecule compound (AO13) on HCV. Virus inhibition was tested by analyzing RNA replication, protein expression, and virus production in virus-infected cells treated with AO13. Treatment with AO13 inhibited virus spread in cell culture and showed a 100-fold reduction in the levels of infectious virus production. AO13 significantly reduced the level of viral RNA contained within cell culture fluids and reduced the cellular levels of HCV core protein, suggesting that the compound might act on a late step in the viral life cycle. Finally, we observed that AO13 did not affect the release of infectious virus from infected cells. Docking studies and molecular dynamics analyses suggested that AO13 might target the NS5B RNA polymerase, however, real-time RT-PCR analyses of cellular levels of HCV RNA showed only an ∼2-fold reduction in viral RNA levels in the presence of AO13. Taken together, this study revealed that AO13 showed consistent, but low-level antiviral effect against HCV, although the mechanism of action remains unclear. IMPORTANCE The discovery of curative antiviral drugs for a chronic disease such as HCV infection has encouraged drug discovery in the context of other viruses for which no curative drugs currently exist. Since we currently face a novel virus that has caused a pandemic, the need for new antiviral agents is more apparent than ever. We describe here a novel compound that shows a modest antiviral effect against HCV that could serve as a lead compound for future drug development against other important viruses such as SARS-CoV-2.
Keywords: HCVcc; JFH-1; antiviral agent; antiviral agents; hepatitis C virus; virus; virus inhibition.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

