Structure and analysis of nanobody binding to the human ASIC1a ion channel
- PMID: 34319232
- PMCID: PMC8318589
- DOI: 10.7554/eLife.67115
Structure and analysis of nanobody binding to the human ASIC1a ion channel
Abstract
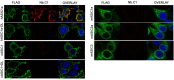
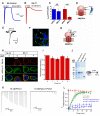
ASIC1a is a proton-gated sodium channel involved in modulation of pain, fear, addiction, and ischemia-induced neuronal injury. We report isolation and characterization of alpaca-derived nanobodies (Nbs) that specifically target human ASIC1a. Cryo-electron microscopy of the human ASIC1a channel at pH 7.4 in complex with one of these, Nb.C1, yielded a structure at 2.9 Å resolution. It is revealed that Nb.C1 binds to a site overlapping with that of the Texas coral snake toxin (MitTx1) and the black mamba venom Mambalgin-1; however, the Nb.C1-binding site does not overlap with that of the inhibitory tarantula toxin psalmotoxin-1 (PcTx1). Fusion of Nb.C1 with PcTx1 in a single polypeptide markedly enhances the potency of PcTx1, whereas competition of Nb.C1 and MitTx1 for binding reduces channel activation by the toxin. Thus, Nb.C1 is a molecular tool for biochemical and structural studies of hASIC1a; a potential antidote to the pain-inducing component of coral snake bite; and a candidate to potentiate PcTx1-mediated inhibition of hASIC1a in vivo for therapeutic applications.
Keywords: ASIC1; MitTx antagonism; Pctx1 potentiation; cryo-electron microscopy; molecular biophysics; nanobody; structural biology.
© 2021, Wu et al.
Conflict of interest statement
YW, ZC, FS, CC No competing interests declared
Figures












References
-
- Chassagnon IR, McCarthy CA, Chin YK-Y, Pineda SS, Keramidas A, Mobli M, Pham V, De Silva TM, Lynch JW, Widdop RE, Rash LD, King GF. Potent neuroprotection after stroke afforded by a double-knot spider-venom peptide that inhibits acid-sensing ion channel 1a. PNAS. 2017;114:3750–3755. doi: 10.1073/pnas.1614728114. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

