Contemporaneous Comparison of Outcomes Among Patients Implanted With a Leadless vs Transvenous Single-Chamber Ventricular Pacemaker
- PMID: 34319383
- PMCID: PMC8319824
- DOI: 10.1001/jamacardio.2021.2621
Contemporaneous Comparison of Outcomes Among Patients Implanted With a Leadless vs Transvenous Single-Chamber Ventricular Pacemaker
Erratum in
-
Change to Open Access Status.JAMA Cardiol. 2021 Nov 1;6(11):1344. doi: 10.1001/jamacardio.2021.3756. JAMA Cardiol. 2021. PMID: 34495292 Free PMC article. No abstract available.
Abstract
Importance: The safety and efficacy of leadless VVI pacemakers have been demonstrated in multiple clinical trials, but the comparative performance of the device in a large, real-world population has not been examined.
Objective: To compare patient characteristics and complications among patients implanted with leadless VVI and transvenous VVI pacemakers.
Design, setting, participants: The Longitudinal Coverage With Evidence Development Study on Micra Leadless Pacemakers (Micra CED) is a continuously enrolling observational cohort study evaluating complications, utilization, and outcomes of leadless VVI pacemakers in the US Medicare fee-for-service population. Patients implanted between March 9, 2017, and December 1, 2018, were identified and included. All Medicare patients implanted with leadless VVI and transvenous VVI pacemakers during the study period were enrolled. Patients with less than 12 months of continuous enrollment in Medicare prior to leadless VVI or transvenous VVI implant and with evidence of a prior cardiovascular implantable electronic device were excluded, leaving 5746 patients with leadless VVI pacemakers and 9662 patients with transvenous VVI pacemakers. Data were analyzed from May 2018 to April 2021.
Exposures: Medicare patients implanted with leadless VVI pacemakers or transvenous VVI pacemakers.
Main outcomes and measures: The main outcomes were acute (30-day) complications and 6-month complications.
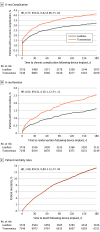
Results: Of 15 408 patients, 6701 (43.5%) were female, and the mean (SD) age was 81.0 (8.7) years. Compared with patients with transvenous VVI pacemakers, patients with leadless VVI pacemakers were more likely to have end-stage kidney disease (690 [12.0%] vs 226 [2.3%]; P < .001) and a higher mean (SD) Charlson Comorbidity Index score (5.1 [3.4] vs 4.6 [3.0]; P < .001). The unadjusted acute complication rate was higher in patients with leadless VVI pacemakers relative to transvenous VVI pacemakers (484 of 5746 [8.4%] vs 707 of 9662 [7.3%]; P = .02). However, there was no significant difference in overall acute complication rates following adjustment for patient characteristics (7.7% vs 7.4%; risk difference, 0.3; 95% CI, -0.6 to 1.3; P = .49). Pericardial effusion and/or perforation within 30 days was significantly higher among patients with leadless VVI pacemakers compared with patients with transvenous VVI pacemakers in both unadjusted and adjusted models (unadjusted, 47 of 5746 [0.8%] vs 38 of 9662 [0.4%]; P < .001; adjusted, 0.8% vs 0.4%; risk difference, 0.4; 95% CI, 0.1 to 0.7; P = .004). Patients implanted with leadless VVI pacemakers had a lower rate of 6-month complications compared with patients implanted with transvenous VVI pacemakers (unadjusted hazard ratio, 0.84; 95% CI, 0.68-1.03; P = .10; adjusted hazard ratio, 0.77; 95% CI, 0.62-0.96; P = .02).
Conclusions and relevance: In this study, despite significant differences in patient characteristics, patients in whom a leadless pacemaker was implanted were observed to have higher rates of pericardial effusion and/or perforation but lower rates of other device-related complications and requirements for device revision at 6 months. Understanding the benefits and risks associated with leadless VVI pacemakers compared with transvenous VVI pacemakers can help clinicians and patients make informed treatment decisions.
Conflict of interest statement
Figures
Comment in
-
Leadless Pacing-Uncertainties Remain About Safety and Efficacy-Reply.JAMA Cardiol. 2022 Mar 1;7(3):361-362. doi: 10.1001/jamacardio.2021.5716. JAMA Cardiol. 2022. PMID: 35080586 No abstract available.
References
-
- Department of Health & Human Services, Centers for Medicare & Medicaid Services. CMS manual system . Accessed April 23, 2021. https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/2017D...
-
- Longitudinal Coverage With Evidence Development Study on Micra Leadless Pacemakers (Micra CED) . ClinicalTrials.gov identifier. NCT03039712. Updated February 21, 2021. Accessed February 21, 2021. https://clinicaltrials.gov/ct2/show/NCT03039712
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

