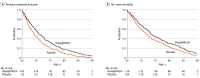
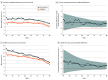
Conflict of Interest Disclosures: Dr Docherty’s employer, the University of Glasgow, was remunerated by AstraZeneca (sponsor of Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure [DAPA-HF]) for his involvement in the DAPA-HF trial, received personal fees from AstraZeneca during the conduct of the study, and received personal fees from Eli Lilly outside the submitted work. Dr Jhund’s employer, the University of Glasgow, was remunerated by AstraZeneca for working on the DAPA-HF and DELIVER trials, received speaker and advisory board fees from AstraZeneca during the conduct of the study, received speaker and advisory board fees from Novartis, his employer, the University of Glasgow, received remuneration from Novartis for working on the PARADIGM-HF and PARAGON-HF trials, received personal fees from Boehringer Ingelheim for serving on the advisory board, received grants from Boehringer Ingelheim outside the submitted work, and is director of GCTP Ltd. Dr Claggett reported receiving consulting fees from AO Biome, Boehringer Ingelheim, Corvia, Myokardia, and Novartis for consulting outside the submitted work. Dr Ferreira reported receiving nonfinancial support from Boehringer Ingelheim. Dr Bengtsson reported receiving personal fees from AstraZeneca as an employee outside the submitted work. Dr Inzucchi reported receiving fees from AstraZeneca for travel costs during the conduct of the study, personal fees from Boehringer Ingelheim, Novo Nordisk, Merck, vTv Therapeutics, Esperion, and Abbott outside the submitted work. Dr Køber reported receiving speakers honorarium from Novo, Novartis, AstraZeneca, and Boehringer outside the submitted work. Dr Kosiborod reported receiving grants from AstraZeneca and Boehringer Ingelheim, and consulting fees from Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Janssen, Merck (diabetes), Novo Nordisk, Sanofi, and Vifor Pharma outside the submitted work. Dr Langkilde reported being a full-time employee and shareholder of AstraZeneca during the conduct of the study. Dr Ponikowski reported receiving personal fees and fees to his institution from participation as an investigator in clinical trials from AstraZeneca during the conduct of the study; personal fees from Boehringer Ingelheim, Vifor Pharma, Amgen, Servier, Novartis, Berlin Chemie, Bayer, Pfizer, Cibiem, Impulse Dynamics, Renal Guard Solutions, Sanofi, BMS, and Respicardia for participation in clinical studies; fees from AbbottVascular as coprincipal investigator of the RESHAPE-HF trial; and personal fees from Radcliffe Group outside the submitted work. Dr Sabatine reported receiving grants from AstraZeneca to the TIMI Study Group at Brigham and Women's Hospital and personal fees from AstraZeneca for consulting during the conduct of the study, personal fees from Althera for consulting, grants from to the TIMI Study Group at Brigham and Women's Hospital, personal fees from Amgen Consulting, personal fees from Anthos Therapeutics for consulting, grants from Bayer to the TIMI Study Group at Brigham and Women's Hospital, personal fees from Bristol-Myers Squibb for consulting, personal fees from CVS Caremark for consulting, grants from Daiichi Sankyo to the TIMI Study Group at Brigham and Women's Hospital, personal fees from DalCor for consulting, personal fees from Dr Reddy's Laboratories for consulting, personal fees from Dyrnamix for consulting, grants from Eisai to the TIMI Study Group at Brigham and Women's Hospital, personal fees from Esperion for consulting, personal fees from IFM Therapeutics for consulting, grants from Intarcia to the TIMI Study Group at Brigham and Women's Hospital, personal fees from Intarcia for consulting, grants from Janssen Research and Development to the TIMI Study Group at Brigham and Women's Hospital, personal fees from Janssen Research and Development for consulting, grants from Medicines Company to the TIMI Study Group at Brigham and Women's Hospital, personal fees from the Medicines Company for consulting, grants from MedImmune to the TIMI Study Group at Brigham and Women's Hospital, personal fees from MedImmune for consulting, grants from to the TIMI Study Group at Brigham and Women's Hospital, personal fees from Merck for consulting, grants from Novartis to the TIMI Study Group at Brigham and Women's Hospital, personal fees from Novartis for consulting, grants from Pfizer to the TIMI Study Group at Brigham and Women's Hospital, and grants from Quark Pharmaceuticals to the TIMI Study Group at Brigham and Women's Hospital outside the submitted work, and is a member of the TIMI Study Group, which has also received institutional research grant support through Brigham and Women's Hospital from Abbott, Regeneron, Roche, and Zora Biosciences. Dr Sjöstrand reported receiving personal fees from AstraZeneca as an employee during the conduct of the study and being an AstraZeneca stockholder outside the submitted work. Dr Solomon reported receiving grants from AstraZeneca to the institution during the conduct of the study; institutional grants from Actelion, Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, BMS, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Lilly, Lone Star Heart, Mesoblast, MyoKardia, NIH/NHLBI, Neurotronik, Novartis, Novo Nordisk, Respicardia, Sanofi Pasteur, and Theracos; and personal fees from Abbott, Actelion, Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi Sankyo, Gilead, GSK, Ironwood, Lilly, Merck, Myokardia, Novartis, Roche, Takeda, Theracos, Quantum Genetics, Cardurion, AoBiome, Janssen, Cardiac Dimensions, Sanofi Pasteur, Tenaya, Dinaqor, Tremeau, CellProThera, Moderna, and American Regent for consulting outside the submitted work. Dr McMurray reported receiving nonfinancial support from AstraZeneca, and his employer, Glasgow University, has been paid by AstraZeneca (which markets dapagliflozin) for his time spent as principal investigator of DAPA-HF and coprincipal investigator of DELIVER and DETERMINE (trials using dapagliflozin) in heart failure fees for meetings and other activities related to these trials. AstraZeneca has also paid Dr McMurray’s for travel and accommodation for these meetings. These payments were made through a consultancy with Glasgow University and Dr McMurray has not received personal payments in relation to this trial/this drug during the conduct of the study. Nonfinancial support was provided by Cytokinetics; Glasgow University has been paid by Cytokinetics for Dr McMurray’s time spent as steering committee member for the ATOMIC-HF, COSMIC-HF, and GALACTIC-HF trials, meetings, and other activities related to these trials. Cytokinetics also paid for travel and accommodation for some of these meetings/activities. These payments were made through a consultancy with Glasgow University, and Dr McMurray has not received personal payments in relation to these trials/this drug. Nonfinancial support was provided by Bayer; Glasgow University has been paid by Bayer for Dr McMurray’s time spent as a steering committee member for the FINEARTS-HF trial (using finerenone in heart failure). These payments were made through a consultancy with Glasgow University, and Dr McMurray has not received personal payments in relation to this trial/drug. Nonfinancial support was provided by Amgen; Glasgow University has been paid by Amgen for Dr McMurray’s time spent as a steering committee member for the GALACTIC-HF trial and meetings related to this trial. Amgen also paid for travel and accommodation for some of these meetings. These payments were made through a consultancy with Glasgow University, and Dr McMurray has not received personal payments in relation to these trials/this drug. Nonfinancial support was provided by Oxford University/Bayer. Glasgow University has been paid by Oxford University (which has received a grant from Bayer, which manufactures acarbose) for Dr McMurray’s time spent as a steering committee member for the ACE trial (using acarbose) and meetings related to this trial. Oxford University also paid for travel and accommodation for some of these meetings. These payments were made through a consultancy with Glasgow University and Dr McMurray has not received personal payments in relation to this trial/this drug. Nonfinancial support was provided by Theracos. Glasgow University has been paid by Theracos for Dr McMurray’s time spent as principal investigator for the BEST trial and meetings related to this trial. Theracos also paid for travel and accommodation for some of these meetings. These payments were made through a consultancy with Glasgow University, and Dr McMurray has not received personal payments in relation to this trial/this drug. Nonfinancial support was provided by DalCor Pharmaceuticals. Glasgow University has been paid by DalCor Pharmaceuticals for Dr McMurray’s time spent as a steering committee member for the Dal-GenE trial and meetings related to this trial. These payments were made through a consultancy with Glasgow University, and Dr McMurray has not received personal payments in relation to this trial/this drug. Nonfinancial support was provided by Merck. Glasgow University has been paid by Merck for Dr McMurray’s time spent on the data safety monitoring committee for the MK-3102 program and for the VICTORIA trial and meetings related to these trials. These payments were made through a consultancy with Glasgow University, and Dr McMurray has not received personal payments in relation to this trial/this drug. Nonfinancial support was provided by GlaxoSmithKline. Glasgow University has been paid by GlaxoSmithKline for Dr McMurray’s time spent as coprincipal investigator for the Harmony-Outcomes trial (albiglutide) and steering committee member for 2 trials, ASCEND-D and ASCEND-ND, using daprodustat, and meetings related to these trials. GlaxoSmithKline also paid for travel and accommodation for some of these meetings. These payments were made through a consultancy with Glasgow University, and Dr McMurray has not received personal payments in relation to these trials/drugs. Nonfinancial support was provided by Bristol Myers Squibb. Glasgow University has been paid by Bristol Myers Squibb for Dr McMurray’s time spent as a steering committee member for the STAND-UP clinical trial (using a nitroxyl [HNO] donor) in heart failure and meetings related to this trial. These payments were made through a consultancy with Glasgow University, and Dr McMurray has not received personal payments in relation to this trial/drug. Nonfinancial support was provided by Vifor Fresenius. Glasgow University has been paid by Kings College Hospital (which received a grant from Kidney Research UK and Vifor Fresenius, which manufactures intravenous iron) for Dr McMurray’s time spent as a steering committee member for the PIVOTAL trial (using intravenous iron) and for running the end point adjudication committee for this trial, as well as meetings related to the PIVOTAL trial. Kings College Hospital also paid for travel and accommodation for some of these meetings. These payments were made through a consultancy with Glasgow University, and Dr McMurray has not received personal payments in relation to this trial/drug. Nonfinancial support was provided by Alnylam. Glasgow University has been paid by Alnylam for Dr McMurray’s time spent on the advisory board committee for the ALN-AGT trial and meetings related to this trial. These payments were made through a consultancy with Glasgow University and Dr McMurray has not received personal payments in relation to this trial/this drug. Nonfinancial support was provided by AbbVie. Glasgow University has been paid by AbbVie (which manufactures atrasentan) for Dr McMurray’s time spent as steering committee member for the SONAR trial (using atrasentan) and meetings related to this trial. AbbVie also paid for travel and accommodation for some of these meetings. These payments were made through a consultancy with Glasgow University, and Dr McMurray has not received personal payments in relation to this trial/this drug. Glasgow University has been paid by Cyclerion for Dr McMurray’s advice about development of praliciguat as a potential therapy for heart failure. Glasgow University has been paid by Cardurion for Dr McMurray’s participation in a company advisory board about development of a phosphodiesterase 9 inhibitor in heart failure. Nonfinancial support was provided by Novartis. Glasgow University has been paid by Novartis for Dr McMurray’s time spent as executive committee member and then coprincipal investigator of the ATMOSPHERE trial, coprincipal investigator of the PARADIGM-HF trial, and executive/steering committee member for the PARACHUTE-HF, PARADISE-MI, and PERSPECTIVE trials (with sacubitril/valsartan) and meetings/presentations related to these trials and aliskiren and sacubitril/valsartan. Novartis also paid for travel and accommodation for some of these meetings. These payments were made through a consultancy with Glasgow University, and Dr McMurray has not received personal payments in relation to this trial/this drug. Glasgow University has also been paid by Novartis for Dr McMurray’s participation in a company advisory board, which meets twice per year and covers the cardiometabolic field. Nonfinancial support was provided by Servier. Glasgow University has been paid by Servier for activities related to Dr McMurray’s role as steering committee member for the GALACTIC-HF trial. These payments were made through a consultancy with Glasgow University, and Dr McMurray has not received personal payments in relation to this trial/ drug. Dr McMurray has received lecture fees from Abbott, Hickma, Sun Pharmaceuticals, and Servier outside the submitted work. No other disclosures were reported.