Delayed adjuvant imatinib in patients with high risk of recurrence of gastrointestinal stromal tumor after radical surgery: a retrospective cohort study
- PMID: 34319443
- PMCID: PMC11800817
- DOI: 10.1007/s00432-021-03749-6
Delayed adjuvant imatinib in patients with high risk of recurrence of gastrointestinal stromal tumor after radical surgery: a retrospective cohort study
Abstract
Purpose: To investigate the impact of delayed adjuvant imatinib on GIST patients with high risk of recurrence.
Method: Adult GIST patients were retrospectively collected from our hospital between 2011 and 2018, and patients having high risk of recurrence were included for subsequent analyses. The primary endpoint was recurrence-free survival (RFS).
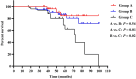
Results: According to the interval between the radical surgery and the beginning of adjuvant imatinib, 222 patients were divided into three groups: group A (≤ 2 months, n = 41), group B (2-≤ 4 months, n = 113), and group C (4-≤ 6 months, n = 68). Univariate, multivariate, and survival analyses all showed that patients in group A had significantly more favorable RFS than those in group C but not group B, and patients taking adjuvant imatinib for over 12 months were also associated with longer RFS comparing to adjuvant imatinib of ≤ 12 months. When stratified by the duration of adjuvant imatinib, no significant differences were found in RFS among groups A, B, and C for adjuvant imatinib of ≤ 12 months. While for adjuvant imatinib of over 12 months, both groups A and B had significantly more favorable RFS than group C, and no significant difference in RFS was found between group A and B.
Conclusion: Delayed postoperative adjuvant imatinib for over 4 months in patients with high risk of recurrence of GIST may lead to worse RFS, and longer treatment with shorter delay has best results.
Keywords: Gastrointestinal stromal tumor; Imatinib; Recurrence-free survival.
© 2021. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
There was no conflict of interest.
Figures



Similar articles
-
Is 3-years duration of adjuvant imatinib mesylate treatment sufficient for patients with high-risk gastrointestinal stromal tumor? A study based on long-term follow-up.J Cancer Res Clin Oncol. 2017 Apr;143(4):727-734. doi: 10.1007/s00432-016-2334-x. Epub 2017 Jan 12. J Cancer Res Clin Oncol. 2017. PMID: 28083710 Free PMC article.
-
Assessing prognostic factors of long-term survival after surgery for colorectal gastrointestinal stromal tumours.Colorectal Dis. 2023 Dec;25(12):2325-2334. doi: 10.1111/codi.16778. Epub 2023 Oct 24. Colorectal Dis. 2023. PMID: 37876119
-
Distinguishing very high-risk patients among high-risk gastrointestinal stromal tumor cases: development and validation of a nomogram based on a multicenter population-based retrospective cohort study.Ann Med. 2025 Dec;57(1):2520896. doi: 10.1080/07853890.2025.2520896. Epub 2025 Jun 20. Ann Med. 2025. PMID: 40539729
-
Imatinib for the treatment of patients with unresectable and/or metastatic gastrointestinal stromal tumours: systematic review and economic evaluation.Health Technol Assess. 2005 Jul;9(25):1-142. doi: 10.3310/hta9250. Health Technol Assess. 2005. PMID: 15985189
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article.
Cited by
-
An additional gastrojejunostomy may reduce the incidence of moderate and severe delayed gastric emptying after distal segmental duodenectomy for gastrointestinal stromal tumors.World J Surg Oncol. 2024 Nov 14;22(1):303. doi: 10.1186/s12957-024-03585-1. World J Surg Oncol. 2024. PMID: 39543700 Free PMC article.
References
-
- Bengio RM, Riva ME, Moiraghi B, Lanari E, Milone J, Ventriglia V, Bullorsky E, Tezanos Pinto M, Murro H, Bianchini M, Larripa I (2011) Clinical outcome of chronic myeloid leukemia imatinib-resistant patients: do BCR-ABL kinase domain mutations affect patient survival? First multicenter Argentinean study. Leuk Lymphoma 52(9):1720–1726 - PubMed
-
- Blanke CD, Demetri GD, von Mehren M, Heinrich MC, Eisenberg B, Fletcher JA, Corless CL, Fletcher CDM, Roberts PJ, Heinz D, Wehre E, Nikolova Z, Joensuu H (2008a) Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 26(4):620–625 - PubMed
-
- Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren M, Benjamin RS, Raymond AK, Bramwell VHC, Baker LH, Maki RG, Tanaka M, Hecht JR, Heinrich MC, Fletcher CDM, Crowley JJ, Borden EC (2008b) Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 26(4):626–632 - PubMed
-
- Cavnar MJ, Seier K, Curtin C, Balachandran VP, Coit DG, Yoon SS, Crago AM, Strong VE, Tap WD, Gonen M, Antonescu CR, Brennan MF, Singer S, DeMatteo RP (2019) Outcome of 1000 patients with gastrointestinal stromal tumor (GIST) treated by surgery in the pre and post-imatinib eras. Ann Surg 273(1):128 - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

