NIR Laser Photobiomodulation Induces Neuroprotection in an In Vitro Model of Cerebral Hypoxia/Ischemia
- PMID: 34319540
- PMCID: PMC8497317
- DOI: 10.1007/s12035-021-02496-6
NIR Laser Photobiomodulation Induces Neuroprotection in an In Vitro Model of Cerebral Hypoxia/Ischemia
Abstract
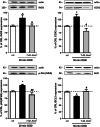
Brain photobiomodulation (PBM) is an innovative treatment for a variety of neurological conditions, including cerebral ischemia. However, the capability of PBM for ischemic stroke needs to be further explored and its mechanisms of action remain currently unclear. The aim of the present research was to identify a treatment protocol capable of inducing neuroprotection and to investigate the molecular mechanisms activated by a dual-wavelength near infrared (NIR) laser source in an organotypic hippocampal slice model of hypoxia/ischemia. Hippocampal slices were exposed to oxygen and glucose deprivation (OGD) for 30 min followed by NIR laser light (fluence 3.71, 7.42, or 14.84 J/cm2; wavelengths 808 nm and 905 nm) delivered immediately or 30 min or 60 min after OGD, in order to establish a therapeutic window. Neuronal injury was assessed by propidium iodide fluorescence 24 h later. Our results show that NIR laser irradiation attenuates OGD neurotoxicity once applied immediately or 30 min after OGD. Western blot analysis of proteins involved in neuroinflammation (iNOS, COX-2, NFkB subunit p65, and Bcl-2) and in glutamatergic-mediated synaptic activity (vGluT1, EAAT2, GluN1, and PSD95) showed that the protein modifications induced by OGD were reverted by NIR laser application. Moreover, CA1 confocal microscopy revealed that the profound morphological changes induced by OGD were reverted by NIR laser radiation. In conclusion, NIR laser radiation attenuates OGD neurotoxicity in organotypic hippocampal slices through attenuation of inflammatory mechanisms. These findings shed light on molecular definition of NIR neuroprotective mechanisms, thus underlining the potential benefit of this technique for the treatment of cerebral ischemia.
Keywords: Glia activation; NIR laser; Neurodegeneration; Organotypic hippocampal slices; Oxygen and glucose deprivation; Photobiomodulation.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Photobiomodulation Therapy Attenuates Hypoxic-Ischemic Injury in a Neonatal Rat Model.J Mol Neurosci. 2018 Aug;65(4):514-526. doi: 10.1007/s12031-018-1121-3. Epub 2018 Jul 22. J Mol Neurosci. 2018. PMID: 30032397 Free PMC article.
-
Neuroprotection by group I mGlu receptors in a rat hippocampal slice model of cerebral ischemia is associated with the PI3K-Akt signaling pathway: a novel postconditioning strategy?Neuropharmacology. 2008 Sep;55(4):509-16. doi: 10.1016/j.neuropharm.2008.06.019. Epub 2008 Jun 19. Neuropharmacology. 2008. PMID: 18606174
-
A novel method for oxygen glucose deprivation model in organotypic spinal cord slices.Brain Res Bull. 2017 Oct;135:163-169. doi: 10.1016/j.brainresbull.2017.10.010. Epub 2017 Oct 17. Brain Res Bull. 2017. PMID: 29054697
-
Organotypic hippocampal slice cultures for studies of brain damage, neuroprotection and neurorepair.Curr Drug Targets CNS Neurol Disord. 2005 Aug;4(4):435-52. doi: 10.2174/1568007054546108. Curr Drug Targets CNS Neurol Disord. 2005. PMID: 16101559 Review.
-
Therapeutic Potentials of Near-Infrared II Photobiomodulation to Treat Cerebrovascular Diseases via Nitric Oxide Signalling.Adv Exp Med Biol. 2024;1463:195-200. doi: 10.1007/978-3-031-67458-7_33. Adv Exp Med Biol. 2024. PMID: 39400823 Free PMC article. Review.
Cited by
-
Chronic administration of prebiotics and probiotics prevent pathophysiological hallmarks of Alzheimer's disease in the cortex of APP/PS1 mice.Front Pharmacol. 2025 May 15;16:1596469. doi: 10.3389/fphar.2025.1596469. eCollection 2025. Front Pharmacol. 2025. PMID: 40444050 Free PMC article.
-
Photobiomodulation for the treatment of neuroinflammation: A systematic review of controlled laboratory animal studies.Front Neurosci. 2022 Sep 20;16:1006031. doi: 10.3389/fnins.2022.1006031. eCollection 2022. Front Neurosci. 2022. PMID: 36203812 Free PMC article.
-
Activation of testosterone-androgen receptor mediates cerebrovascular protection by photobiomodulation treatment in photothrombosis-induced stroke rats.CNS Neurosci Ther. 2024 Feb;30(2):e14574. doi: 10.1111/cns.14574. CNS Neurosci Ther. 2024. PMID: 38421088 Free PMC article.
-
Pulsed Nd:YAG laser therapy accelerates fracture healing in a rat femoral osteotomy model.Bone Joint Res. 2025 May 2;14(5):376-388. doi: 10.1302/2046-3758.145.BJR-2024-0285.R2. Bone Joint Res. 2025. PMID: 40312041 Free PMC article.
-
Hypoxia/Ischemia-Induced Rod Microglia Phenotype in CA1 Hippocampal Slices.Int J Mol Sci. 2022 Jan 26;23(3):1422. doi: 10.3390/ijms23031422. Int J Mol Sci. 2022. PMID: 35163344 Free PMC article.
References
-
- The top 10 causes of death. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death. Accessed 21 Apr 2021
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

