Nutrient Deficiency and an Algicidal Bacterium Improved the Lipid Profiles of a Novel Promising Oleaginous Dinoflagellate, Prorocentrum donghaiense, for Biodiesel Production
- PMID: 34319787
- PMCID: PMC8436737
- DOI: 10.1128/AEM.01159-21
Nutrient Deficiency and an Algicidal Bacterium Improved the Lipid Profiles of a Novel Promising Oleaginous Dinoflagellate, Prorocentrum donghaiense, for Biodiesel Production
Abstract
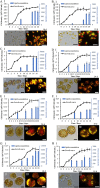
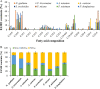
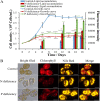
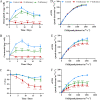
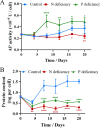
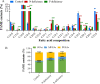
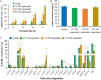
The lipid production potentials of 8 microalgal species were investigated. Among these 8 species, the best strain was a dominant bloom-causing dinoflagellate, Prorocentrum donghaiense; this species had a lipid content of 49.32% ± 1.99% and exhibited a lipid productivity of 95.47 ± 0.99 mg liter-1 day-1, which was 2-fold higher than the corresponding values obtained for the oleaginous microalgae Nannochloropsis gaditana and Phaeodactylum tricornutum. P. donghaiense, which is enriched in C16:0 and C22:6, is appropriate for commercial docosahexaenoic acid (DHA) production. Nitrogen or phosphorus stress markedly induced lipid accumulation to levels surpassing 75% of the dry weight, increased the C18:0 and C17:1 contents, and decreased the C18:5 and C22:6 contents, and these effects resulted in decreases in the unsaturated fatty acid levels and changes in the lipid properties of P. donghaiense such that the species met the biodiesel specification standards. Compared with the results obtained under N-deficient conditions, the enhancement in the activity of alkaline phosphatase of P. donghaiense observed under P-deficient conditions partly alleviated the adverse effects on the photosynthetic system exerted by P deficiency to induce the production of more carbohydrates for lipogenesis. The supernatant of the algicidal bacterium Paracoccus sp. strain Y42 culture lysed P. donghaiense without decreasing its lipid content, which resulted in facilitation of the downstream oil extraction process and energy savings through the lysis of algal cells. The Y42 supernatant treatment improved the lipid profiles of algal cells by increasing their C16:0, C18:0, and C18:1 contents and decreasing their C18:5 and C22:6 contents, which is favorable for biodiesel production. IMPORTANCE This study demonstrates the high potential of Prorocentrum donghaiense, a dominant bloom-causing dinoflagellate, for lipid production. Compared with previously studied oleaginous microalgae, P. donghaiense exhibit greater potential for practical application due to its higher biomass and lipid contents. Nutrient deficiency and the algicidal bacterium Paracoccus sp. strain Y42 improved the suitability of the lipid profile of P. donghaiense for biodiesel production. Furthermore, Paracoccus sp. Y42 effectively lysed algal cells, which facilitates the downstream oil extraction process for biodiesel production and results in energy savings through the lysing of algal cells. This study provides a more promising candidate for the production of docosahexaenoic acid (DHA) for human nutritional products and of microalgal biofuel as well as a more cost-effective method for breaking algal cells. The high lipid productivity of P. donghaiense and algal cell lysis by algicidal bacteria contribute to reductions in the production cost of microalgal oil.
Keywords: DHA; Prorocentrum donghaiense; algicidal bacteria; biodiesel production; lipid accumulation; nutrient starvation.
Figures

References
-
- Borowitzka MA. 1995. Microalgae as sources of pharmaceuticals and other biologically active compounds. J Appl Phycol 7:3–15. 10.1007/BF00003544. - DOI
-
- Milano J, Ong HW, Masjuki HH, Chong WT, Lam MK, Loh PK, Vellayan V. 2016. Microalgae biofuels as an alternative to fossil fuel for power generation. Renew Sustain Energy Rev 58:180–197. 10.1016/j.rser.2015.12.150. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

