Beneficial effects of dietary supplementation with green tea catechins and cocoa flavanols on aging-related regressive changes in the mouse neuromuscular system
- PMID: 34319911
- PMCID: PMC8351677
- DOI: 10.18632/aging.203336
Beneficial effects of dietary supplementation with green tea catechins and cocoa flavanols on aging-related regressive changes in the mouse neuromuscular system
Abstract
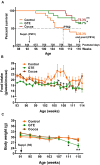
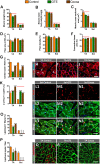
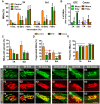
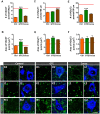
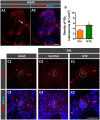
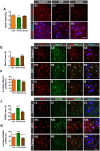
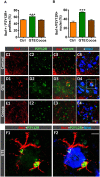
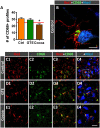
Besides skeletal muscle wasting, sarcopenia entails morphological and molecular changes in distinct components of the neuromuscular system, including spinal cord motoneurons (MNs) and neuromuscular junctions (NMJs); moreover, noticeable microgliosis has also been observed around aged MNs. Here we examined the impact of two flavonoid-enriched diets containing either green tea extract (GTE) catechins or cocoa flavanols on age-associated regressive changes in the neuromuscular system of C57BL/6J mice. Compared to control mice, GTE- and cocoa-supplementation significantly improved the survival rate of mice, reduced the proportion of fibers with lipofuscin aggregates and central nuclei, and increased the density of satellite cells in skeletal muscles. Additionally, both supplements significantly augmented the number of innervated NMJs and their degree of maturity compared to controls. GTE, but not cocoa, prominently increased the density of VAChT and VGluT2 afferent synapses on MNs, which were lost in control aged spinal cords; conversely, cocoa, but not GTE, significantly augmented the proportion of VGluT1 afferent synapses on aged MNs. Moreover, GTE, but not cocoa, reduced aging-associated microgliosis and increased the proportion of neuroprotective microglial phenotypes. Our data indicate that certain plant flavonoids may be beneficial in the nutritional management of age-related deterioration of the neuromuscular system.
Keywords: aging; cocoa; green tea; neuromuscular system; sarcopenia.
Conflict of interest statement
Figures

Similar articles
-
Motoneuron deafferentation and gliosis occur in association with neuromuscular regressive changes during ageing in mice.J Cachexia Sarcopenia Muscle. 2020 Dec;11(6):1628-1660. doi: 10.1002/jcsm.12599. Epub 2020 Jul 20. J Cachexia Sarcopenia Muscle. 2020. PMID: 32691534 Free PMC article.
-
Green tea extract attenuates muscle loss and improves muscle function during disuse, but fails to improve muscle recovery following unloading in aged rats.J Appl Physiol (1985). 2015 Feb 1;118(3):319-30. doi: 10.1152/japplphysiol.00674.2014. Epub 2014 Nov 20. J Appl Physiol (1985). 2015. PMID: 25414242 Free PMC article.
-
Green tea extract containing enhanced levels of epimerized catechins attenuates scopolamine-induced memory impairment in mice.J Ethnopharmacol. 2020 Aug 10;258:112923. doi: 10.1016/j.jep.2020.112923. Epub 2020 May 1. J Ethnopharmacol. 2020. PMID: 32360798
-
Recommendations for Development of New Standardized Forms of Cocoa Breeds and Cocoa Extract Processing for the Prevention of Alzheimer's Disease: Role of Cocoa in Promotion of Cognitive Resilience and Healthy Brain Aging.J Alzheimers Dis. 2015;48(4):879-89. doi: 10.3233/JAD-150536. J Alzheimers Dis. 2015. PMID: 26402120 Review.
-
Safety assessment of green tea based beverages and dried green tea extracts as nutritional supplements.Toxicol Lett. 2017 Aug 5;277:104-108. doi: 10.1016/j.toxlet.2017.06.008. Epub 2017 Jun 24. Toxicol Lett. 2017. PMID: 28655517 Review.
Cited by
-
Decoding Neuromuscular Disorders: The Complex Role of Genetic and Epigenetic Regulators.Genes (Basel). 2025 May 23;16(6):622. doi: 10.3390/genes16060622. Genes (Basel). 2025. PMID: 40565514 Free PMC article. Review.
-
Exploring the Therapeutic Potential of Green Tea (Camellia sinensis L.) in Anti-Aging: A Comprehensive Review of Mechanisms and Findings.Mini Rev Med Chem. 2025;25(5):403-424. doi: 10.2174/0113895575331878240924035332. Mini Rev Med Chem. 2025. PMID: 39377377 Review.
-
Dietary flavonoid intake is negatively associated with accelerating aging: an American population-based cross-sectional study.Nutr J. 2024 Dec 20;23(1):158. doi: 10.1186/s12937-024-01052-x. Nutr J. 2024. PMID: 39707398 Free PMC article.
-
A Comprehensive Review of Pathological Mechanisms and Natural Dietary Ingredients for the Management and Prevention of Sarcopenia.Nutrients. 2023 Jun 3;15(11):2625. doi: 10.3390/nu15112625. Nutrients. 2023. PMID: 37299588 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

