Computational modelling unveils how epiblast remodelling and positioning rely on trophectoderm morphogenesis during mouse implantation
- PMID: 34320001
- PMCID: PMC8318228
- DOI: 10.1371/journal.pone.0254763
Computational modelling unveils how epiblast remodelling and positioning rely on trophectoderm morphogenesis during mouse implantation
Abstract
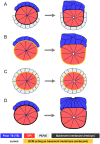
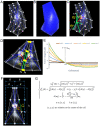
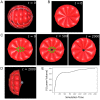
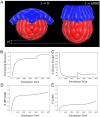
Understanding the processes by which the mammalian embryo implants in the maternal uterus is a long-standing challenge in embryology. New insights into this morphogenetic event could be of great importance in helping, for example, to reduce human infertility. During implantation the blastocyst, composed of epiblast, trophectoderm and primitive endoderm, undergoes significant remodelling from an oval ball to an egg cylinder. A main feature of this transformation is symmetry breaking and reshaping of the epiblast into a "cup". Based on previous studies, we hypothesise that this event is the result of mechanical constraints originating from the trophectoderm, which is also significantly transformed during this process. In order to investigate this hypothesis we propose MG# (MechanoGenetic Sharp), an original computational model of biomechanics able to reproduce key cell shape changes and tissue level behaviours in silico. With this model, we simulate epiblast and trophectoderm morphogenesis during implantation. First, our results uphold experimental findings that repulsion at the apical surface of the epiblast is essential to drive lumenogenesis. Then, we provide new theoretical evidence that trophectoderm morphogenesis indeed can dictate the cup shape of the epiblast and fosters its movement towards the uterine tissue. Our results offer novel mechanical insights into mouse peri-implantation and highlight the usefulness of agent-based modelling methods in the study of embryogenesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Trophectoderm mechanics direct epiblast shape upon embryo implantation.Cell Rep. 2021 Jan 19;34(3):108655. doi: 10.1016/j.celrep.2020.108655. Cell Rep. 2021. PMID: 33472064 Free PMC article.
-
Donor embryonic stem cells displace host cells of 8-cell-stage chimeras to the extra-embryonic lineages by spatial crowding and FGF4 signalling.Development. 2025 Jun 15;152(12):dev204518. doi: 10.1242/dev.204518. Epub 2025 Jun 24. Development. 2025. PMID: 40446208 Free PMC article.
-
Self-organizing properties of mouse pluripotent cells initiate morphogenesis upon implantation.Cell. 2014 Feb 27;156(5):1032-44. doi: 10.1016/j.cell.2014.01.023. Epub 2014 Feb 13. Cell. 2014. PMID: 24529478 Free PMC article.
-
[Epiblast and primitive endoderm cell specification during mouse preimplantation development: a combination between biology and mathematical modeling].Med Sci (Paris). 2016 Feb;32(2):192-7. doi: 10.1051/medsci/20163202013. Epub 2016 Mar 2. Med Sci (Paris). 2016. PMID: 26936177 Review. French.
-
Computational models for the dynamics of early mouse embryogenesis.Int J Dev Biol. 2019;63(3-4-5):131-142. doi: 10.1387/ijdb.180418gd. Int J Dev Biol. 2019. PMID: 31058292 Review.
Cited by
-
Spectral decomposition unlocks ascidian morphogenesis.Elife. 2025 Jun 16;13:RP94391. doi: 10.7554/eLife.94391. Elife. 2025. PMID: 40522321 Free PMC article.
-
Modeling Epiblast Shape in Implanting Mammalian Embryos.Methods Mol Biol. 2022;2490:281-296. doi: 10.1007/978-1-0716-2281-0_20. Methods Mol Biol. 2022. PMID: 35486253
-
Epithelial-Mesenchymal Transition Drives Three-Dimensional Morphogenesis in Mammalian Early Development.Front Cell Dev Biol. 2021 Feb 11;9:639244. doi: 10.3389/fcell.2021.639244. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33644076 Free PMC article.
-
Effective mechanical potential of cell-cell interaction explains three-dimensional morphologies during early embryogenesis.PLoS Comput Biol. 2023 Aug 7;19(8):e1011306. doi: 10.1371/journal.pcbi.1011306. eCollection 2023 Aug. PLoS Comput Biol. 2023. PMID: 37549166 Free PMC article.
-
Digitize your Biology! Modeling multicellular systems through interpretable cell behavior.bioRxiv [Preprint]. 2023 Nov 5:2023.09.17.557982. doi: 10.1101/2023.09.17.557982. bioRxiv. 2023. Update in: Cell. 2025 Jul 25:S0092-8674(25)00750-0. doi: 10.1016/j.cell.2025.06.048. PMID: 37745323 Free PMC article. Updated. Preprint.
References
-
- Kojima Y, Tam OH, Tam PP. Timing of developmental events in the early mouse embryo. In: Seminars in cell developmental biology. vol. 34. Elsevier; 2014. p. 65–75. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

